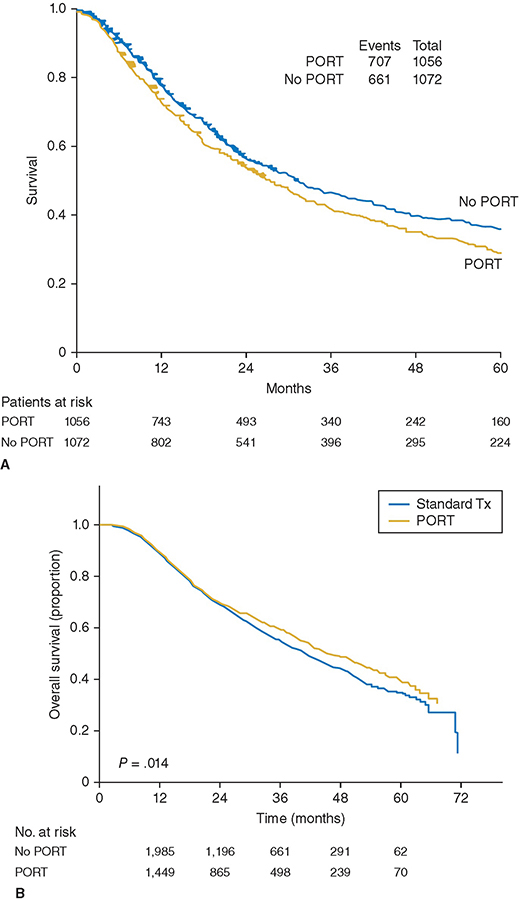
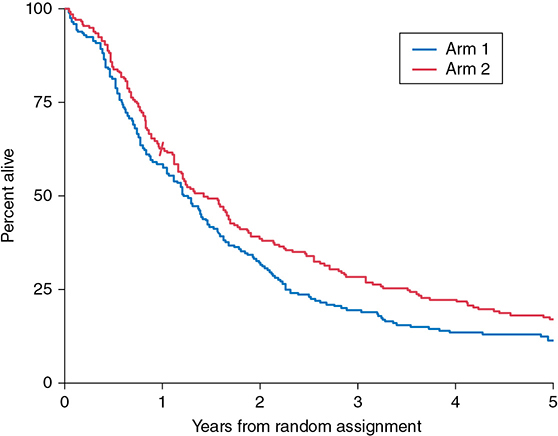
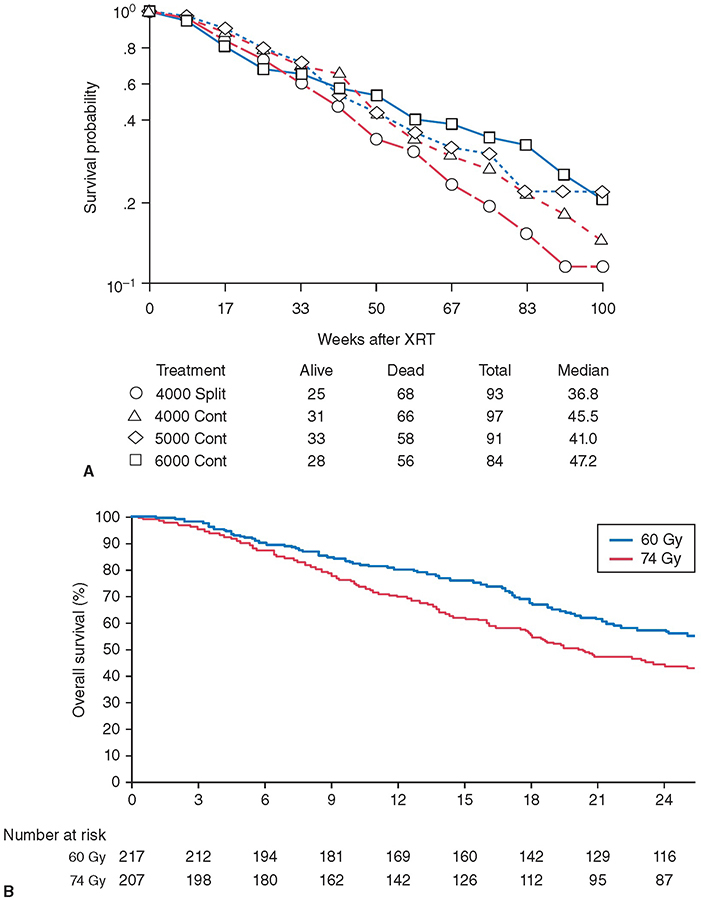
A 60-year-old male has a 6-cm right apical lung mass that invades the chest wall. He has chronic obstructive pulmonary disease (COPD) and smokes 1 pack of cigarettes per day. The mass is biopsied by computed tomographic (CT)–guided core needle and is positive for adenocarcinoma of the lung. The pathology shows non–small cell lung cancer (NSCLC) of the lung. Positron emission tomography (PET) shows one ipsilateral PET-positive, non-bulky medastinal lymph node that is pathologically positive on mediastinoscopy. Magnetic resonance images (MRI) of the brain and neck are negative for metastases or extension into the plexus. Learning Objectives: 1. What is the role of surgery in stage III NSCLC? 2. What surgical techniques are available to resect lung cancer invading adjacent organs? 3. How are Pancoast tumors of the lung treated? Locally advanced NSCLC includes tumors that invade the surrounding structures, including the chest wall, vertebrae, great vessels, diaphragm, and structures of the superior sulcus. Treatment typically involves multimodal therapy, with surgical resection providing the best chance at improving 5-year survival. In this chapter, the surgical management of locally advanced NSCLC is discussed. Pancoast tumors were originally described by Henry Pancoast, the first president of the American Board of Radiology.1 Also known as superior sulcus tumors, these tumors invade the apical chest wall and surrounding structures, including the brachial plexus, sympathetic chain, subclavian artery and vein, vertebrae, spinal cord, clavicle, and ribs.2 Approximately 90% of all Pancoast tumors are NSCLCs2 and are classified according to the tumor, node, metastasis (TNM) staging system as T3 or T4 lesions depending on the extent of invasion. The National Comprehensive Cancer Network (NCCN) guidelines can be used to determine treatment strategies. Treatment options are multimodal and include a combination of chemotherapy, radiation therapy, and surgical excision.3 However, because less than 5% of all NSCLCs are Pancoast tumors, no prospective head-to-head randomized controlled trials comparing treatment strategies have been conducted.2 The NCCN guidelines recommend neoadjuvant concurrent chemoradiation for T3N0-1 and possibly resectable T4N0-1 Pancoast tumors. Standard regimens include platinum-based chemotherapy along with 45- to 54-Gray (Gy) radiation given in 1.8- to 2-Gy fractions.3 Induction chemoradiotherapy is associated with an improved 5-year survival rate, complete pathologic response, and R0 resection rate compared to the historical treatment regimen of preoperative radiotherapy followed by surgery. Furthermore, trimodal therapy has been shown to downstage tumors.2 Surgical resection is the mainstay of treatment of Pancoast tumors, with the goal of R0 resection, including the upper lobe with one rib with attached intercostal muscles below the inferior margin of the tumor and all invaded structures en bloc.4 Absolute contraindications to surgical resection include extrathoracic metastases, N2 disease, and invasion of the trachea, esophagus, spinal canal, or brachial plexus above the T1 nerve root because of the functional loss that occurs with resection of the lower trunk.5,6 Resection can be approached anteriorly or posteriorly depending on structures involved. Most Pancoast tumors are found in the posterior compartment of the thoracic inlet5 where neural structures are located6 and are accessed through a posterolateral approach.5 Anterior lesions extending into the subclavian vessels5 or first rib6 are approached anteriorly.5,6 Several anterior approaches exist, including anterior transcervical-transthoracic, hemiclamshell, modified hemiclamshell, and Masoaka incision.4–9 Pancoast tumors can also be resected using minimally invasive techniques.10,11 Lesions involving the anterior sulcus can be resected using a transcervical incision, which was first described by Dartevelle.12 The patient is placed supine, and a large L-shaped cervicotomy is made along the sternocleidomastoid muscle and extended laterally to the deltopectoral groove just inferior to the clavicle.8,12 Full exposure of the thoracic inlet is obtained by dividing the sternal head of the sternocleidomastoid muscle and the clavicular attachment of pectoralis major and resecting the medial clavicle.7,8 The subclavian vein is exposed and resected if invaded by tumor.8 The phrenic nerve is then identified along the anterior scalene muscle and preserved if uninvolved by tumor. The anterior scalene is divided at its insertion on the first rib. The phrenic nerve is resected as well as the anterior scalene at its attachment on the transverse processes of the third through sixth cervical vertebrae if either is grossly involved.6,8 The subclavian artery is then exposed and dissected away from the tumor if possible. Resection and revascularization with end-to-end anastomosis or with polytetrafluoroethylene (PTFE) graft is required if the tumor invades the wall of the subclavian artery.7,8 At its insertion on the first rib, the middle scalene is divided to expose the brachial plexus. If tumor involvement extends beyond the first rib, it is also divided at its attachment on the transverse processes of the second through seventh cervical vertebrae. Divide prevertebral muscles and sympathetic chain and ganglia from the seventh cervical and first thoracic vertebral bodies and then transect the first thoracic nerve root lateral to the intervertebral foramen.8 Finally, the first rib is disarticulated, and ribs two and three are resected.7,8 Upper lobectomy is then performed and removed en bloc.12 The hemiclamshell or trapdoor incision extends a partial upper sternotomy into an anterior thoracotomy.8,11 An oblique incision along the anterior border of the sternocleidomastoid just superior to the midsternal notch is extended into an upper median sternotomy down to the third or fourth intercostal space and then extended laterally along that intercostal space to the anterior axillary line.8 After the sternum and internal mammary artery are transected, the chest wall is retracted, thereby exposing the superior sulcus and mediastinum.8 The superior vena cava (SVC) is dissected superiorly to the subclavian vein. The ribs are resected posterolaterally and at the costochondral junctions. The subclavian vein, artery, and brachial plexus are then resected if involved by tumor in the same fashion as in the transcervical approach,9 and lobectomy is performed. If optimal exposure of the thoracic inlet structures is not achieved with chest wall retraction alone, then the clavicle can also be resected.8 The hemiclamshell approach can be modified to include resection of the costoclavicular ligament and first costal cartilage, creating a larger sternocostal flap and better exposure of the hilar structures as well as the thoracic inlet and posterior chest wall.4 This is an anterior trans-sternal approach described by Masoaka to provide extended exposure of the anterior part of the superior sulcus.11 A transverse incision is made at the base of the neck just superior to the clavicle, followed by an upper median sternotomy to the fourth intercostal space.8,11 The posterolateral approach was first described by Shaw and Paulson to allow sufficient exposure of the vertebral bodies, transverse processes, and brachial plexus.6,11 However, it may be difficult to achieve R0 resection with this approach if local invasion of the tumor extends to the vasculature above these structures.6 A posterolateral thoracotomy is made with the patient in the lateral decubitus position and extended posteriorly just medial to the scapula to the level of the seventh cervical vertebra.8 The trapezius, levator scapulae, rhomboid major, and rhomboid minor muscles are divided posteriorly while the latissimus dorsi is retracted anteriorly, and the serratus anterior and posterior muscles are divided to allow for retraction of the scapula.8 Chest wall resection is then carried out with a 4-cm anterior margin and an inferior margin extending 1 rib and intercostal muscle beneath the gross inferior margin of the tumor.8 Scalene muscles are divided, and the involved ribs are transected. The first rib is disarticulated from the costovertebral joint to expose the brachial plexus, and the first thoracic nerve root is subsequently transected lateral to the intervertebral foramen. The subclavian artery and vein are then resected if necessary in the same fashion as described. The sympathetic chain and ganglia are transected along with up to one-fourth of the corresponding vertebral bodies if involved by tumor. With the specimen attached, upper lobectomy is completed. Chest wall reconstruction is not needed as the scapula and clavicle close off the defect.8 The thoracoscopic approach to both pulmonary resection and chest wall resection for Pancoast tumors is also used. The thoracoscope allows for optimal views of the apical chest wall and superior sulcus and for a more accurate evaluation of the correct level of chest wall resection. Furthermore, the minimally invasive approach requires only a small incision for en bloc resection and permits the preservation of the musculature overlying the area of resection, resulting in less postoperative pain.10,11 Tumors that invade the chest wall are at least T3 by definition and account for roughly 5% of all lung cancers.13 Chest wall invasion generally arises from the local spread of peripheral tumors.13 Surgical resection is the mainstay of initial therapy for all T3 tumors with chest wall invasion.3 NCCN guidelines recommend neoadjuvant chemotherapy or concurrent chemoradiation for all T4N0-1 (stage IIIA) tumors invading the chest wall.3 As in the resection of Pancoast tumors, lateral margins of tumors invading the chest wall should be 4 cm, and superior and inferior margins should include 1 intact rib with intercostal muscles.13 Similarly, reconstruction is not necessary for defects deep to the scapula. However, reconstruction is needed to cover large defects to preserve the structure and function of the chest wall. The chest wall can be reconstructed using a variety of prosthetic materials, including polypropylene mesh, PTFE mesh, soft tissue xenografts, and titanium plates.13 Tumors that invade the vertebra are also classified as T4. Surgical resection involves the removal of at least three hemivertebrae, one above and one below tumor invasion, followed by spinal fixation.7 Vertebrae can be resected first or after the resection of other involved structures and lobectomy. In the latter, the previously described transcervical approach is first completed. Prior to closure, the trachea and esophagus are retracted, and the anterior longitudinal ligament is dissected to expose the lower cervical and upper thoracic vertebrae. The transcervical incision is closed, and the patient is then placed prone; a vertical midline incision from C7 to T4 is made followed by unilateral laminectomies. Involved nerve roots are divided, and the corresponding vertebral bodies are transected in the midline, which allows for en bloc removal of the specimen. The spine is then fixed with hooks and screws.7 Alternatively, spinal resection with fixation can be done prior to chest wall resection and lobectomy without exposure of the tumor itself.14 The NCCN guidelines recommend initial en bloc surgical resection of tumors invading the great vessels and the heart without mediastinal lymph node involvement or metastatic disease.3 Resection of NSCLC invading the aorta can be performed with or without cardiopulmonary bypass.15,16 Depending on the size of the defect, it can be closed primarily, with a prosthetic patch graft, or replaced with a prosthetic interposition graft.17 When cardiopulmonary bypass is used, resection and reconstruction of the invaded segment is performed after aortic cross-clamping.15 Shunting from the ascending aorta and the descending aorta distal to the lesion can also be used, which preserves blood flow to the spinal cord, abdominal viscera, and lower extremities.17 Alternatively, the placement of non-fenestrated endovascular stents is also used to avoid bypass and shunting altogether. The stent can be placed at the same time as surgical resection or several days prior.15,18 The stent forms a framework that maintains flow both during the resection and after, does not require defect coverage, and should be placed at least 3 cm from the expected aortic defect.15,18 After endograft placement, a hemiclamshell or thoracotomy incision can be used for the lobectomy or pneumonectomy, with en bloc resection of the tumor and all invaded structures.17,18 Non–small cell lung cancer invasion into the SVC is exceedingly rare.19 An endovascular stent can be placed in patients with unresectable disease and/or SVC syndrome to provide relief of symptoms caused by obstruction.19 Surgical resection is indicated in patients without N2 or metastatic disease. If the brachiocephalic vein is involved, then a sternotomy incision is made; otherwise, a thoracotomy incision provides adequate exposure to the SVC.20,21 Extent of invasion cannot be assessed without opening the pericardium.20 If less than one-fourth of the circumference of the SVC is involved, a side clamp can be used to resect and primarily repair the defect.21,22 A prosthetic patch or autologous pericardial patch is used to repair defects up to 50% of the diameter.21,22 For defects greater than 50%, a ringed prosthetic graft or autologous spiral saphenous vein graft is placed after circumferential resection of the involved segment.20–22 Cross-clamping of the SVC is avoided if the graft is placed between the brachiocephalic vein and the right atrial appendage rather than a brachiocephalic vein and the SVC.21 Lobectomy or pneumonectomy is then performed and en bloc resection is complete. Despite a poor prognosis for localized NSCLCs invading the left atrium, surgical resection remains the gold standard.23 Because the right pulmonary veins are shorter, invasion of the left atrium is more commonly seen with right-sided tumors.24 A thoracotomy incision is made, and the pericardium is opened. The involved atrial wall is clamped and resected. The stump can be repaired primarily or with a patch graft if the reduction in atrial volume affects hemodynamics.25 Lobectomy or pneumonectomy is then performed to complete en bloc resection. Tumors with diaphragmatic involvement are now classified as T4 (previously T3) in the latest TNM staging classification because of their poorer prognosis.26 These tumors should be considered potentially curable by resection; however, literature regarding these outcomes is limited and often single center.27–32 Within the English literature, Weksler et al. first reported on the postsurgical outcomes of NSLC invading the diaphragm. In their series of 8 patients, all surgical specimens showed invasion of the diaphragmatic musculature, and all 4 patients with N2 disease died of recurrent disease, highlighting the importance of mediastinal staging and chemotherapy in this patient population.28 In a more recent series, Galetta et al. reported a series of 19 patients in which depth of invasion to the muscular diaphragm and again, nodal involvement, were associated with a worse prognosis for survival. In this study, patients with pathologically confirmed N2 disease received induction chemotherapy and were only offered surgery if the tumor showed a response or no progression.27 After resection of the diaphragm, closure of the defect can usually be done primarily, with bridging prosthetic rarely required.27,28,30 In one series, prosthetic replacement of the diaphragmatic defect was associated with improved survival, supporting a hypothesis of interrupting the drainage of the diaphragmatic lymphatics to the abdomen; therefore, the authors advocated for a larger margin of resection.30 Because of the difficulty in preoperatively diagnosing diaphragmatic involvement, thoracoscopic investigation and MRI have both been advocated prior to determination of therapy in cases where diaphragmatic involvement is suspected.27,28,31 The NSCLC tumor invading the diaphragm is a prime example of the importance of a multidisciplinary team approach to cancer staging and treatment where input from surgery, pathology, oncology and radiology are used to optimize patient outcomes. The surgical management of locally-advanced NSCLC is complex due to extrapulmonary invasion by the tumor. Because of the aggressive nature of the disease, large randomized controlled trials have not been conducted comparing the various treatment strategies. Nevertheless, it is becoming more widely accepted that surgical resection, in the absence of mediastinal node involvement or other contraindications described previously, in conjunction with other treatment modalities can provide better outcomes. A 72-year-old male has a 4-cm lung mass biopsied by core needle biopsy. He has COPD and smokes 1 pack of cigarettes per day. The biopsy pathology shows NSCLC of the lung. PET shows bulky mediastinal lymph nodes that are pathologically positive on mediastinoscopy. Brain MRI brain is negative. Learning Objectives: 1. What is the role of radiation therapy in locally advanced NSCLC? 2. How do you combine it with other treatment modalities? Radiation therapy is an essential modality in treatment of locally advanced NSCLC. It can be used in a variety of sequences with surgery and chemotherapy depending on the cancer stage, individual patient characteristics, and institutional preference. It is estimated that 84% of stage II and 66% of stage III lung cancer cases require radiotherapy.33 This area of lung cancer treatment is rapidly evolving as new technologies and combinations with systemic agents are being developed. Tumor resectability must be determined in a multidisciplinary discussion prior to starting treatment.34 An experienced thoracic surgeon is needed as there are no definite rules that determine resectability, and the patients need to be approached on a case-by-case basis. This is particularly so given that the staging system does not completely correlate with surgical resectability, and that newer surgical techniques may enable resection of previously unresectable disease. In general, counterindications to resection include significant mediastinal fat invasion; invasion of vital mediastinal structures such as heart, great vessels, aorta, esophagus, vertebrae, or trachea; malignant pleural or pericardial effusion; N2 disease that is bulky or with extracapsular spread; N3 disease; and distant metastases.35 Surgical resection offers superior staging ability as nodal metastases are not always detectable with certainty despite CT scanning, especially when evaluating subcarinal lymph nodes.36 After surgery alone as definitive treatment, survival is poor. A 3-year survival was found to be 9%.36 For stage IIIB disease, the 5-year survival was only 5%.37 Because of this, combined modality trials were designed in order to improve distant and local failures and thus improve survival. To improve the outcomes of locally advanced NSCLC patients treated with surgery, neoadjuvant therapy was explored. The goal was to lead to tumor downstaging, increasing the chance of a complete resection and to improve local control and survival. A randomized controlled trial published in 1972 demonstrated inferior survival in patients with lung adenocarcinoma treated with preoperative radiotherapy. Radiation doses were between 30 and 60 Gy, with most patients receiving 40 to 50 Gy, followed by surgery; this was compared to surgery alone.38 In the first 12 months, the difference was statistically significant, but even at longer follow-up, while not reaching statistical significance, the survival in the radiotherapy group was 12.5% compared to 21% in a surgery-only one. Notably, older radiotherapy techniques were used in the study, which pre-dated CT-guided radiation therapy. Therefore, the outcomes with modern radiotherapy techniques would be expected to be superior due to more accurate radiation delivery, as illustrated in Figure 14-1. Figure 14-1. A. Technique used prior to CT-guided radiotherapy from a 1980s publication of RTOG 7301.65 Treatment fields are illustrated for different tumor locations. Without using CT guidance, it was difficult to clearly visualize the tumor and avoid normal structures. Moreover, the use of posterior spinal cord blocks likely resulted in missing some of mediastinal lymph nodes affected by cancer. (Reproduced with permission from Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Cancer. 1980;45:2744-2753. © American Cancer Society.) B. CT-guided radiotherapy results in improved accuracy of treatment. With IMRT, there is additional improvement in conformity of the high-dose region to the target volume and normal organ sparing compared to 3-dimentional conformation radiation therapy. The colored regions in the pictures show the areas of radiation dose deposition. The legend in the upper right indicates the percentage of the prescribed dose delivered to each colored area. (Reproduced with permission from Reference 118 by Diwanji TP, Mohindra P, Vyfhuis M, et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl Lung Cancer Res. 2017;6(2):131-147.) Given the lack of clinical improvement with neoadjuvant radiation therapy alone, neoadjuvant radiation therapy combined with chemotherapy was investigated. In a prospective trial, Southwest Oncology Group (SWOG) 8805, concurrent cisplatin-etoposide with 45 Gy in 25 fractions of radiotherapy followed by surgery resulted in an encouraging 3-year survival rate of 27% and 24% for stage IIIA and stage IIIB NSCLC, respectively.39 The toxicity was acceptable as well. Another prospective multicenter trial confirmed these findings in stage IIIB disease using cisplatin and docetaxel with 44 Gy in 22 fractions followed by definitive surgery.40 A higher dose of radiotherapy to 61.2 Gy concurrently with chemotherapy was subsequently evaluated in a prospective phase 2 study, RTOG 0229, in N2-3 stage III NSCLC patients to sterilize the mediastinal lymph nodes.41 The results were promising, showing 63% of patients achieved mediastinal nodal clearance and 2-year overall survival of 54% and 2-year progression-free survival of 33%. The toxicity was acceptable with grade 3 or 4 hematologic, pulmonary, and gastrointestinal toxicity occurring in 35%, 23% and 14% of patients, respectively. With the success of chemoradiation given prior to surgery, the question becomes whether surgery is necessary in every case of an operable locally advanced lung cancer. In patients with stage IIIA NSCLC, EORTC 08941 showed that overall survival did not differ between surgery and radiotherapy after induction chemotherapy with cisplatin, carboplatin, and one other chemotherapy drug as illustrated in Figure 14-2.42 More distant recurrences occurred after surgery and more local failures after radiation therapy. The radiotherapy group had less than 1% of esophageal and 4% of pulmonary toxicity and 7% late esophageal or pulmonary toxicities with 1 death. Postoperative mortality ranged from 9% for left-sided pneumonectomy to 0% after lobectomy. Therefore, radiation therapy after chemotherapy is the preferred locoregional treatment to surgery after chemotherapy due to its low morbidity and mortality. The phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE) trial of stage III NSCLC patients evaluated induction chemotherapy with cisplatin-paclitaxel followed by concurrent chemoradiotherapy with cisplatin-vinorelbine with radiation therapy to 45 Gy in 1.5-Gy fractions given twice a day followed by either a radiotherapy boost to 65-71 Gy or surgical resection.43 There was no difference in overall survival or progression-free survival between the two arms. Therefore, both treatment modalities are acceptable in stage III disease. Figure 14-2. Data from EORTC 08941 showing equivalent survival in stage IIIA-N2 NSCLC patients who received induction chemotherapy followed by either surgery or radiotherapy. (Reproduced with permission by van Meerbeeck JP, Kramer GWPM, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99(6):442-450.) Another prospective randomized controlled trial, INT-0139, compared radiotherapy to 45 Gy plus cisplatin-etoposide chemotherapy with surgery to a group that did not receive surgery but underwent continued radiotherapy to 61 Gy.44 In the surgery group, there was a better progression-free survival (12.8 vs. 10.5 months) and disease-free survival at 5 years (22% vs. 11%). While there was no difference in overall survival, the surgery group had more treatment-related deaths (8% vs. 2%). The toxicity was comparable. This study helped establish that chemotherapy with radiotherapy with or without resection is an acceptable option for stage T1-3pN2M0 NSCLC. Superior sulcus tumors are a special consideration in NSCLC due to their unique location, making surgery more challenging. Neoadjuvant chemoradiation in attempt to downstage the tumor prior to resection is therefore an attractive option for superior sulcus tumors. A prospective phase 2 study, JCOG 9806, showed that a trimodality approach with mitomycin, vindesine, cisplatin, 45 Gy of radiotherapy followed by surgical resection is safe and effective in superior sulcus tumors.45 The disease-free and overall survival rates at 5 years were 45% and 56%, respectively. Another prospective trial INT 0160 using cisplatin-etoposide followed by 45 Gy of radiotherapy followed by surgery and two more cycles of additional adjuvant chemotherapy resulted in an encouraging 55% 2-year survival and 70% rate of complete response.46 Postoperative radiation therapy (PORT) is a well-studied area of locally advanced lung cancer treatment but continues to be a clinical challenge. A PORT meta-analysis trialists group in 1998, using older radiation techniques, evaluated all stages of NSCLC from 9 studies including 2,128 patients.47 It showed that PORT was detrimental to overall and progression-free survival in patients with early stage completely resected NSCLC and should not be used, as illustrated in Figure 14-3A. Figure 14-3. A. PORT Meta-analysis Trialists Group data showing worsening survival in patients of all stages receiving postoperative radiotherapy. B. Patients with pathologic N2 NSCLC who underwent complete resection and adjuvant chemotherapy had a statistically significant improvement in overall survival with PORT in an National Cancer Database (NCDB) analysis. (Reproduced with permission by Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol. 2015;33(8):870-876. Copyright © 2015 American Society of Clinical Oncology.) The role of PORT in stage III was not clear. The Surveillance, Epidemiology, and End Results (SEER) database retrospective review of 7,495 patients with NSCLC stage II and III disease demonstrated that PORT increased overall survival in patients with N2 disease but decreased the survival of N0 and N1 patients.48 A 2008 retrospective analysis of the Adjuvant Navelbine International Trialist Association (ANITA) trial of 840 NSCLC patients with stage IB to IIIA disease compared outcomes of patients treated with and without PORT.49 Of note, patients were originally randomized to either observation or adjuvant vinorelbine-cisplatin. The data were retrospectively analyzed to compare the outcomes of PORT and no radiotherapy. The patients who received PORT had improved median survival in both observation and chemotherapy arms in pN2 disease (23.8 vs. 47.4 in chemotherapy and 12.7 vs. 22.7 in observation) and in the observation arm alone in pN1 disease, 25.9 versus 50.2 months (while it was detrimental in the chemotherapy arm, 23.8 vs. 47.4 months). A National Cancer Database analysis of 4,483 patients with N2 NSCLC revealed that there was a 5-year overall survival advantage to PORT compared to no PORT—39.3% compared to 34.8%50—as shown in Figure 14-3B. While there is a lack of consensus, patients whose tumors have not been completely resected (R1/R2 resection) and probably individuals with N2 disease may benefit from PORT.34 As surgical resection is not possible in these patients, the question was whether radiation therapy alone would provide adequate outcomes in locally advanced disease. Cancer and Leukemia Group B (CALGB 8433) investigated whether sequential chemoradiation therapy performed better than radiation therapy alone in stage III lung cancer.51 The combination of cisplatin-vinblastine chemotherapy with 60-Gy radiation in 30 fractions of radiation therapy resulted in median survival improvement of 4.1 months compared to radiation therapy to 60 Gy in 30 fractions alone. While the addition of chemotherapy did not appear to affect local recurrence, there was a significant improvement in distant failure-free survival. Based on this, chemotherapy should not be omitted. However, for patients who are too frail to tolerate both chemotherapy and radiotherapy, one or the other modality may be used. For unresectable disease, various sequences of chemotherapy and radiation therapy have been investigated. In RTOG 9410, stage III NSCLC patients underwent either definitive concurrent or sequential chemoradiotherapy.52 In both cases, radiation therapy was delivered to 63-Gy radiation, and chemotherapy consisted of cisplatin and vinblastine. A concurrent arm showed an improved 5-year survival from 10% to 16%, as illustrated in Figure 14-4. There was greater acute grade 3 or higher toxicity with the concurrent regimen, but there was no difference in late toxicity. Figure 14-4. Results of RTOG 9410 showing improved survival in patients who underwent concurrent chemoradiotherapy (Arm 2) compared to sequential delivery of chemotherapy and radiation therapy (Arm 1). (Reproduced with permission by Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452-1460.) Another 3-arm prospective trial, LAMP (Locally Advanced Multimodality Protocol), evaluated induction chemotherapy followed by radiotherapy, induction chemotherapy followed by concurrent chemoradiation, and concurrent chemoradiation followed by additional chemotherapy in stage III disease.53 The paclitaxel-carboplatin combination was used for chemotherapy, and radiation therapy was administered to 63 Gy. The concurrent chemoradiation followed by chemotherapy arm resulted in the best overall survival (16.3 compared to 13 and 12.7 months) at the expense of a higher rate of esophagitis with radiotherapy. Because of this, concurrent chemoradiation therapy is recommended for patients with inoperable stage III NSCLC. However, patients who are frail and are unable to tolerate concurrent therapy may be treated with the sequential approach. Prophylactic cranial irradiation (PCI) has been investigated as NSCLC commonly metastases to the brain. In a prospective study (RTOG 0214), patients previously treated definitively with no evidence of disease progression were assigned to either PCI to 30 Gy in 15 fractions or observation.54 While patients treated with PCI were 2.5 times more likely to develop brain metastases, the 1-year overall survival and disease-free survival did not differ. A more recent prospective study, NVALT-11/DLCRG-02 evaluated the quality of life and compared the development of symptomatic brain metastases between PCI and observation in stage III patients treated with chemoradiotherapy with or without surgery. While PCI decreased the proportion of patients with symptomatic brain metastases from 27% to 7%, the quality of life was decreased 3 months post-PCI, memory impairment was worse (30% compared to 8%), and cognitive disturbances were present more often (19% compared to 3%) when PCI was not used. Because of this, PCI is not used in NSCLC due to its toxicity and lack of sufficient clinical benefit. Both acute and late toxicity are a major concern with radiation therapy. To minimize the risk of developing these, normal organ dose constraints have been developed. Studies, including QUANTEC55,56 and RTOG studies such as RTOG 0617,57 are used to determine which radiation doses are safe for certain organs at risk. These include spinal cord, esophagus, brachial plexus, heart/pericardium, great vessels, tracheal and proximal bronchi, ribs, skin, and stomach depending on the exact location of the tumor. The toxicities depend on the exact location of the tumor, patient’s comorbidities, prior radiation therapy, and individual genetic susceptibility. Radiation-induced adverse events include acute skin reaction, fatigue, dysphagia, odynophagia, cough, and myelosuppression. Late toxicities include radiation pneumonitis, lung fibrosis, brachial plexopathy, Lhermitte syndrome, radiation myelitis, esophageal fibrosis and strictures, pericarditis, and secondary malignancies.58 Advanced radiation treatment techniques such as intensity-modulated radiation therapy (IMRT) have been shown to reduce the chance of developing these problems.59 General principles of radiation therapy include determining the tumor location, finding how much tissue to include in the radiation field, avoiding delivery of radiation to the normal structures, and maximizing the precision of radiation treatment delivery. The gross tumor volume (GTV) is determined from the CT scan in treatment planning combined with the information obtained from a PET scan. Biopsy-proven lymph nodes or any nodes larger than 15 mm on CT are included in the radiation field. GTV is expanded to an internal target volume (ITV) to provide a margin for target motion. Planning target volume (PTV) is subsequently re-created, which has a 1- to 1.5-cm margin to account for setup uncertainties and patient movement. The margin can be decreased by immobilization, motion management, and image-guided radiation therapy (IGRT).60,61 Radiation therapy interruptions and dose reductions should be avoided if possible. Omitting irradiation of elective nodes in NSCLC stage I-IIIB was investigated in a retrospective study.62 Only the involved lymph nodes by the tumor were included in the radiation treatment field. Elective nodal control was 92.4%, and local control was 51% at 2 years. As nodal failure was rare and only treating the involved field resulted in a reduced radiation dose to the normal tissues, involved-field radiation therapy without treating the elective nodes is the standard of care. A prospective study of 200 patients with inoperable stage III NSCLC involved randomization of patients to cisplatin concurrently with radiotherapy to either 68-74 Gy for involved-field irradiation only compared to 60-64 Gy for elective nodal irradiation as well as involved-field irradiation.63 Two-year overall survival was higher in the involved-field irradiation group, 39.4% compared to 25.6%. A 5-year local control was also better with involved-field irradiation, 51% compared to 36%. Moreover, omission of elective nodal irradiation resulted in less toxicity, manifested as radiation pneumonitis in 17% of involved-field irradiation compared to 29% in the other group. However, it is unknown whether the decrease in local control and survival could be mainly because a lower radiation dose was used in the elective nodal irradiation group. However, there are circumstances under which irradiation of elective lymph node regions may be considered. This depends on various variables related to the tumor, patient, staging, and treatment-related issues and should be evaluated on a case-by-case basis.64 The total radiation dose must be chosen such that the tumor or the remaining microscopic disease is adequately addressed without causing excessive toxicity. In a definitive chemoradiation or radiation-alone setting, the dose of 60 to 70 Gy in 30-35 fractions is the most commonly used.34 The dose of 60 Gy in 30 fractions was shown to be superior to 40 or 50 Gy in the RTOG 7301 study.65 The 3-year overall survival was improved from 6% in patients treated to 40 Gy, 10% in those treated to 50 Gy, to 15% in patients who received 60 Gy, as shown in Figure 14-5A. Dose escalation to 74 Gy appears to be detrimental compared to 60 Gy in patients treated with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab in a prospective trial (RTOG 0617), as shown in Figure 14-5B.57 The higher radiation dose resulted in lower mean and overall survival (29 vs. 20 months and 67 vs. 54%). Surprisingly, fewer local regional failures and better quality of life were noted in the 60-Gy arm. Figure 14-5. A. Improvement in survival was achieved with doses up to 60 Gy in a classic study, RTOG 7031.65 B. Worsening of overall survival was shown when the radiation dose was escalated to 74 Gy from 60 Gy. (Reproduced with permission by Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187-199. Copyright © 2015 Elsevier.) For postoperative cases, 60-70 Gy are typically given for gross residual tumor, 54-60 Gy to extracapsular nodal extension and positive margin, and 50-54 Gy to negative margin. The preoperative radiotherapy dose usually ranges from 45 to 54 in 25-27 fractions. It is important to note that institutional protocols and experience vary, and that the patients treated in high-volume centers have superior outcomes with radiotherapy.34,61 Radiation fractionation is important as different tumor types benefit from various treatment schemes depending on the biology of a specific cancer. In NSCLC, using modified fractionation of radiation therapy such as 54 Gy total given 3 times a day instead of daily radiation treatments to a total of 60 Gy was explored. A CHART (continuous, hyperfractionated, accelerated radiotherapy) randomized trial demonstrated a 9% absolute improvement in overall survival at 2 years.66 This was postulated to be due to accelerated delivery of therapy preventing cancer cellular repopulation from a more protracted regimen. A meta-analysis looking at 10 trials with a total of 2,000 patients revealed that while there was a significant overall survival benefit at 5 years with modified fractionation compared to conventional fractionation (10.8% compared to 8.3%, respectively), this came at the expense of greater acute esophageal toxicity (odds ratio of 2.44) but not the other types of toxicity.67 Overall, further research is needed to identify whether modified fractionation has benefits over a once-a-day schedule. Intensity-modulated radiation therapy is an advanced mode of high-precision radiotherapy that uses computer-controlled linear accelerators to deliver radiation to a tumor.58 The technique uses the accuracy of CT scanning to define the 3-dimensional target volume and normal structures and delivers treatments in a non-uniform intensity of the radiation beams using computerized inverse planning. Because variable radiation intensity is generated across each beam, in contrast to the uniform intensity used in other radiotherapy techniques, very complex plans can be constructed. Using this technology, tumors can be targeted precisely while avoiding normal structures. A secondary analysis of a prospective randomized study (RTOG 0617) of definitive chemoradiation for stage III NSCLC compared outcomes of patients treated with IMRT to 3-dimensional conventional radiation therapy (3D-CRT).59 IMRT resulted in decreased incidence of high-grade radiation pneumonitis, 3.5% compared to 7.9%. The cardiac radiation dose was also significantly lower with IMRT compared to 3D-CRT. The tumor control and overall survival were similar. Other advanced technologies, such as 4-dimensional CT (4D-CT) scan, PET/CT simulation, respiratory motion management, and proton therapy may be appropriate to deliver curative radiation therapy safely. No randomized trials showed their superiority over older techniques, but non-randomized comparisons showed promising toxicity and survival outcomes. For instance, a phase 2 study of carboplatin-paclitaxel chemotherapy with proton beam radiation therapy to 74-Gy relative biological effectiveness resulted in medial overall survival of 26.5 months, with toxicity outcomes that compared favorably with prior conventional radiation therapy.68 Further trials prospectively comparing modalities and evaluating cost-effectiveness are needed. Radiotherapy to NSCLC is a rapidly changing field. Various clinical trials are currently open to accrual. One emerging technology is adaptive radiation therapy. This technique allows for adjustment in the radiation treatment plan as the patient is undergoing radiation therapy as the tumor shrinks and lungs may undergo changes.69 RTOG 1106 is prospective trial using 18F-fludeoxyglucose (FDG)–PET/CT to modify the radiotherapy treatment plan.70 Modern treatment regimens that maximize radiation dose delivery to areas that are the most likely to bear tumor and minimize radiation dose to normal tissues are currently being evaluated prospectively. One example of this is the ongoing phase 3 randomized trial Lung ART (Adjuvant Radiation Therapy) that uses information from the thoracic CT and PET scan obtained prior to surgery as well as the description of the mediastinal exploration and histopathological results for treatment planning.71 There is of course great interest in using immunotherapy for every cancer type. This is clearly the case with NSCLC, especially after durvalumab has been shown to improved progression-free survival after chemoradiation therapy of stage III patients.72 Radiotherapy and immunotherapy combinations are currently being investigated in multiple studies, such as the PARIS trial, which is a phase 1 study of pembrolizumab in combination with radiotherapy in locally advanced NSCLC.73 Another area of interest is hypofractionation. University of Texas Southwestern is conducting a trial comparing 60 Gy in 15 fractions compared to the standard 60-66 Gy in 30-33 fractions in patients with poor functional status.74 Using fewer radiation fractions decreases the number of times the patients need to come to the clinic and may result in better outcomes. Proton therapy for lung cancer has been evaluated in retrospective studies showing promising results. PRONTOX is a prospective trial comparing proton to photon radiation therapy given to 66 Gy (or Gray radiological equivalent in the case of proton therapy).75 The primary aim of this study is to show a decrease of radiation pneumonitis and esophagitis by proton therapy. Asymptomatic brain metastases have been described for patients with locally advanced NSCLC with guidelines from the US NCCN recommending contrasted brain MRI as part of pretreatment staging for patients with stage II-IV NSCLC.3 Patients with locally advanced NSCLC have a high risk for developing brain metastases during the course of their disease.76 Subsequently, the development of new neurologic symptoms warrants central nervous system (CNS) imaging. Given this proclivity for intracranial failure, the RTOG attempted to perform a phase 3 study investigating if PCI was associated with improved survival in patients with locally advanced NSCLC.54 Though this study demonstrated PCI significantly reduced the rate of brain metastasis development at 1 year (p = .004; 7.7% vs. 18.0% for PCI vs. observation), no significant difference in overall survival was found, and PCI is not currently standard of care for patients with locally advanced NSCLC. A 65-year-old white male with a past medical history of COPD presents with persistent cough and weight loss. He has a 60 pack-year smoking history. Chest CT reveals a 5-cm right lower lobe mass with 3-cm, single-station mediastinal lymphadenopathy. Endobronchial ultrasound–guided fine-needle aspiration biopsy confirms adenocarcinoma. No other metastatic site is identified. Learning Objectives: 1. What is the best approach to the management of stage III NSCLC? 2. What is the benefit of chemotherapy in stage III NSCLC? 3. What is the role of immunotherapy in stage III NSCLC? Stage III NSCLC is a heterogeneous group of diseases, and the optimal management of these patients remains challenging. An individualized treatment strategy should be sought from a multidisciplinary assessment for the best outcome in these patients. As a general rule, T4N0-3 or T1-4N3 tumors are unresectable, and definitive concurrent chemoradiation is the standard of care. In resectable tumors, adjuvant chemotherapy has shown a survival benefit. A subset of patients with N2 disease can benefit from induction chemotherapy with or without radiation therapy followed by surgery. Several phase 3 randomized studies and meta-analyses have established the survival benefit of adjuvant chemotherapy in resected stage III NSCLC. The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis of 4,584 patients from 5 large randomized trials evaluated the benefit of postoperative cisplatin-based chemotherapy in stage I-III NSCLC. At the median follow up period of 5.2 years, there was 5.4% absolute survival benefit favoring adjuvant chemotherapy in the overall study population (hazard ratio [HR] 0.89, 95% CI 0.82-0.96, p = .005). In this pooled analysis, approximately 1,200 patients had stage III disease and derived the most survival benefit from adjuvant chemotherapy. The HR for stage III was 0.83 (95% CI 0.72-0.94). The benefit of chemotherapy increased with better performance status (PS) and may be detrimental for PS of 2.77 The largest study included in the LACE meta-analysis was the International Adjuvant Lung Cancer Trial. A total of 1,867 patients underwent randomization, with enrollment of 39% pathologically confirmed stage III disease. Patients were randomly assigned to either 3 or 4 cycles of cisplatin-based chemotherapy or observation. The subset of patients with stage III NSCLC had a significantly higher 5-year overall survival rate (37.4% vs. 29.9%, p < .05).78
14
NON–SMALL CELL LUNG CANCER TREATMENT: LOCALLY ADVANCED
SURGERY FOR LOCALLY ADVANCED NON–SMALL CELL LUNG CANCER
PANCOAST TUMORS
PREOPERATIVE CHEMORADIATION
OVERVIEW OF SURGERY
ANTERIOR APPROACH
Anterior Transcervical-Transthoracic
Hemiclamshell/Trapdoor Incision
Modified Hemiclamshell
Masoaka Incision
POSTERIOR APPROACH
Posterolateral
MINIMALLY INVASIVE APPROACH
LOWER CHEST WALL RESECTION
RECONSTRUCTION OPTIONS
Vertebral Body Resection
Resection of Great Vessel
Aorta
Superior Vena Cava
Atrium
Diaphragm
SURGERY AS PART OF MULTIDISCIPLINARY CARE
RADIATION THERAPY FOR LOCALLY ADVANCED NON–SMALL CELL LUNG CANCER
LOCALLY ADVANCED RESECTABLE NSCLC
PREOPERATIVE RADIOTHERAPY FOLLOWED BY SURGERY
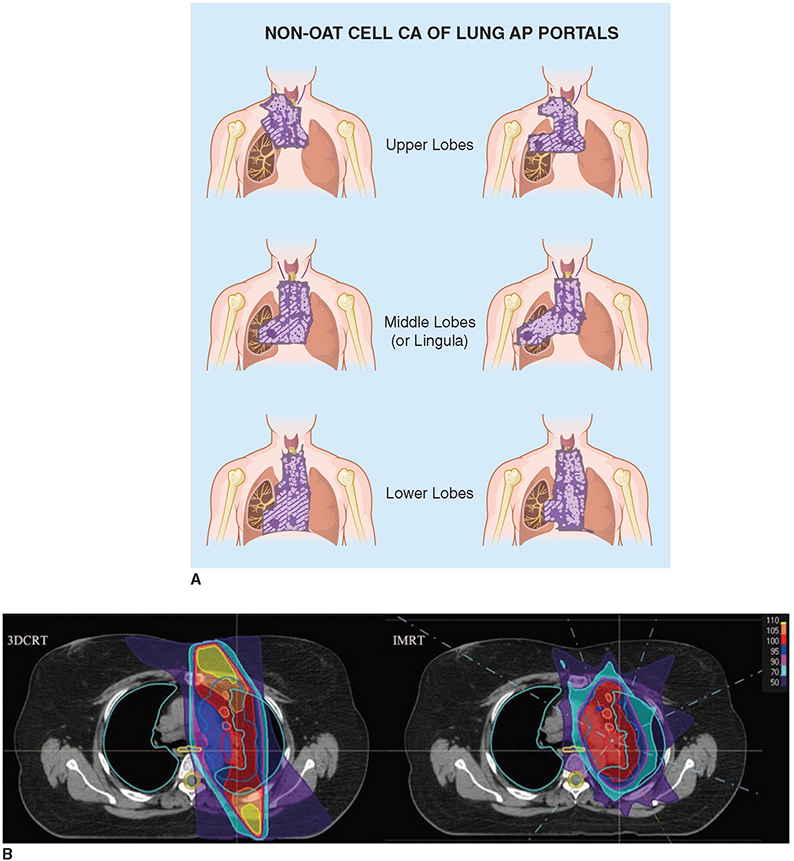
PREOPERATIVE CHEMORADIOTHERAPY FOLLOWED BY SURGERY
INDUCTION CHEMOTHERAPY FOLLOWED BY SURGERY COMPARED TO CHEMORADIOTHERAPY OR RADIOTHERAPY ALONE
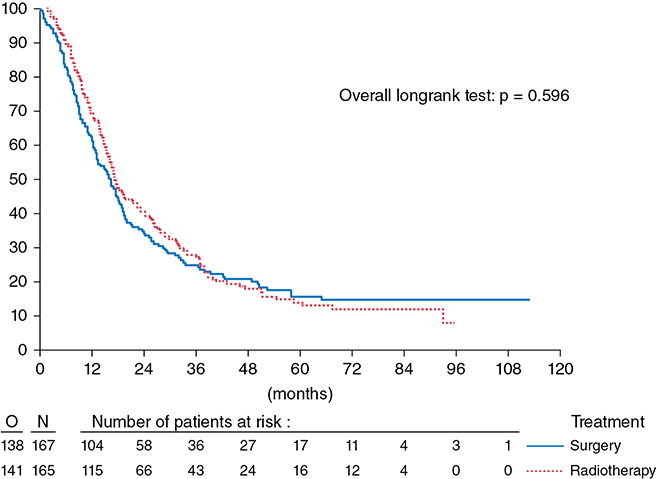
INDUCTION CHEMORADIOTHERAPY FOLLOWED BY SURGERY COMPARED TO DEFINITIVE CHEMORADIOTHERAPY
POSTOPERATIVE RADIATION THERAPY
LOCALLY ADVANCED UNRESECTABLE NSCLC
Definitive Radiotherapy
Sequential Compared to Concurrent Chemoradiotherapy
PROPHYLACTIC CRANIAL IRRADIATION
RADIATION TOXICITY
RADIATION TECHNIQUES
Radiation Treatment Planning
Radiation Treatment Volume
Radiation Dose
Radiation Fractionation
Intensity-Modulated Radiation Therapy
Other Radiation Techniques
ONGOING STUDIES
CENTRAL NERVOUS SYSTEM RADIATION
CHEMOTHERAPY FOR LOCALLY ADVANCED NON–SMALL CELL LUNG CANCER
RESECTABLE STAGE III NSCLC
Adjuvant Chemotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree