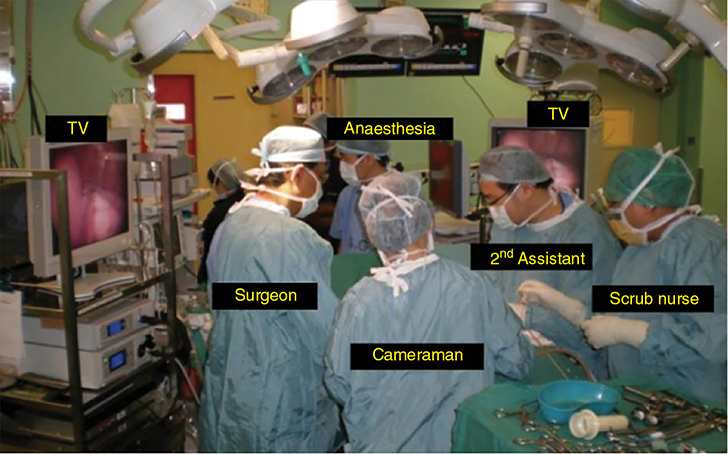
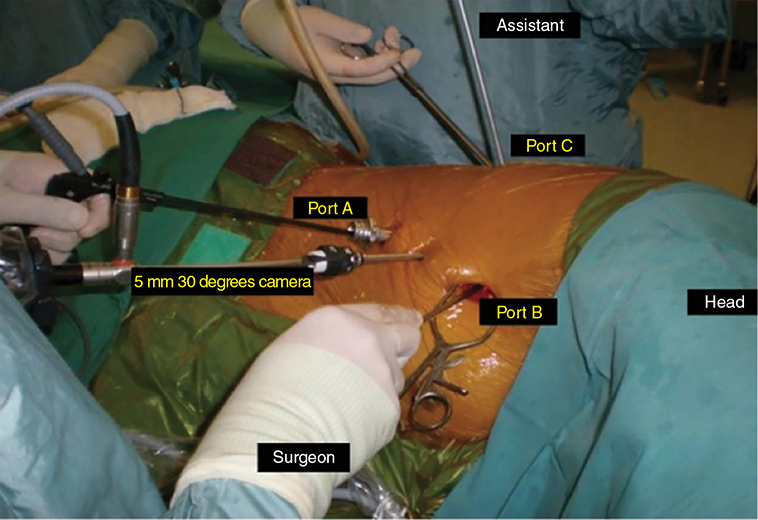
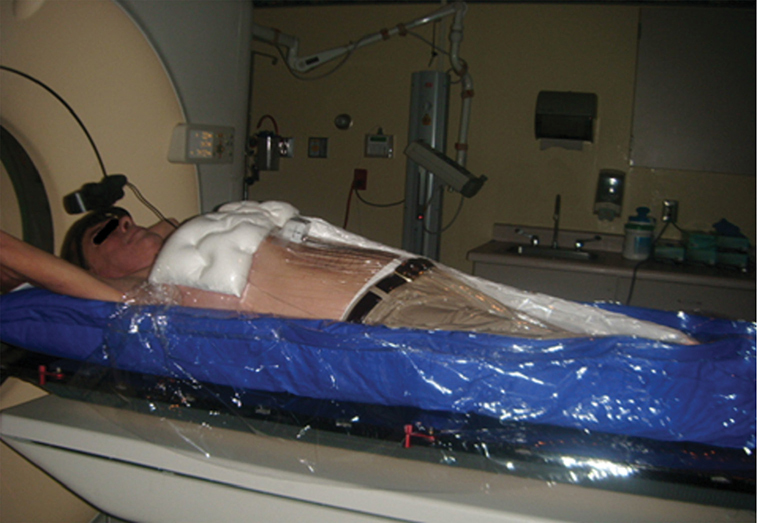
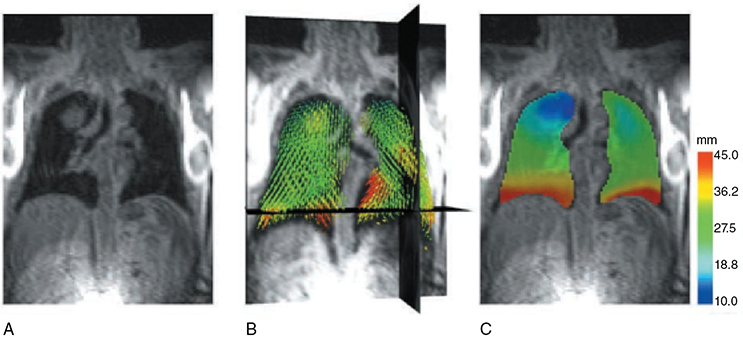
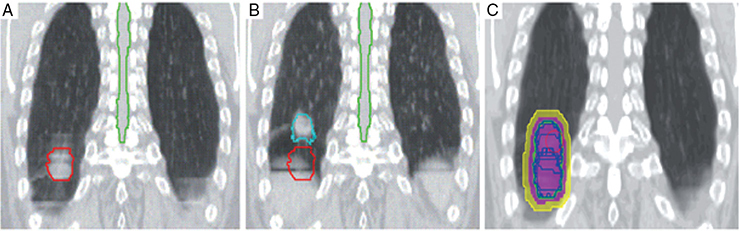
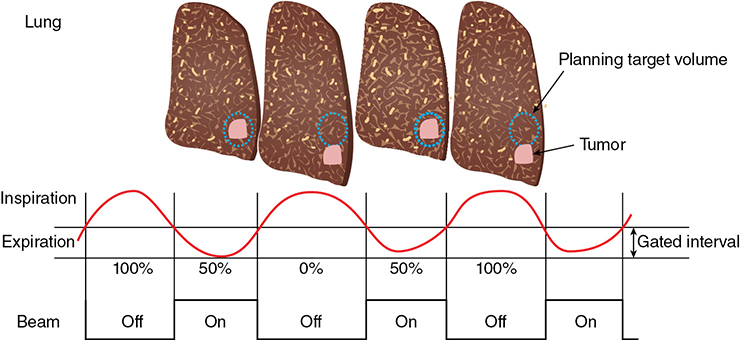
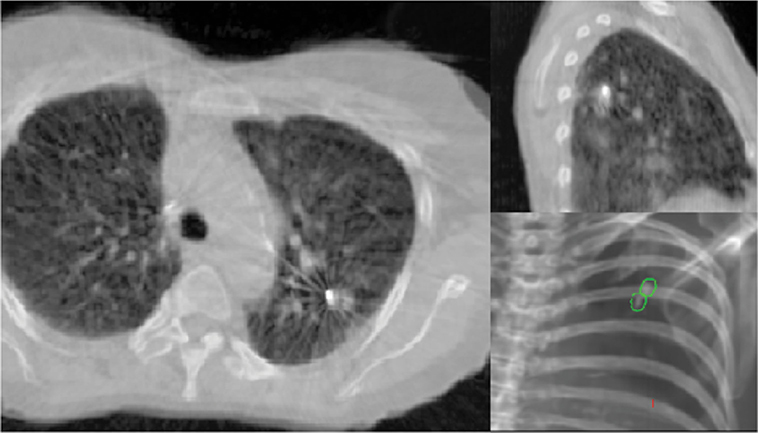
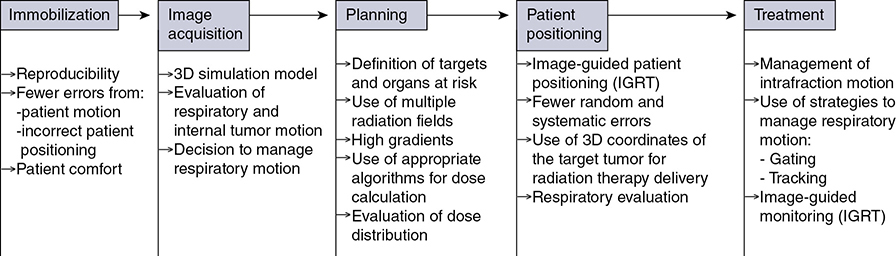
A 62-year-old African American male presents to his primary care physician for routine health maintenance. He has diabetes mellitus (DM) controlled on oral medications and hypertension (HTN), for which he takes hydrochlorothiazide and lisinopril. He has a 50 pack-year smoking history, so his physician orders low-dose chest computed tomography (CT), which shows a 2.1-cm mass in the left upper lobe area. His laboratory work is normal. He sees a pulmonology and undergoes endobronchial ultrasound (EBUS)–guided biopsy, which shows adenocarcinoma. The patient wonders if he will need surgery, radiation, chemotherapy, or all three. Learning Objectives: 1. How do you determine the best modality for biopsy of a lung nodule? 2. Which lymph nodes are sampled with a mediastinoscopy? 3. Which tests should be done prior to lung cancer resection? Assessment of a newly found lung nodule should begin with a history and physical to determine the extent of the disease, focusing especially on areas that may imply presence of metastatic disease. Questioning should be directed toward possible symptoms within and outside of the pulmonary system, including pain within the long bones and vertebrae, new lesions of the skin, and focal neurological findings, such as headache, nausea, vomiting, or seizure. Other suggestions toward metastatic disease include constitutional symptoms such as anorexia, unintentional weight loss, and general malaise. Physical examination warrants attention to palpable lymph nodes, especially the cervical and supraclavicular basins; muscle wasting; and chest auscultation. Routine laboratory studies searching for paraneoplastic syndromes include complete blood count, basic metabolic panel, calcium, and the hepatic enzymes glutamic oxaloacetic transaminase and alkaline phosphatase. Further assessment of a lung nodule should continue with non-invasive staging of disease using advanced imaging. The status of intrathoracic nodal disease will be the patient’s major determining factor when discussing treatment options. If disease has not spread from the primary tumor to mediastinal or subcarinal lymph nodes, surgical resection would be the preferred choice. If a patient does not already had a thin-cut chest CT, a scan with intravenous contrast should be performed for elucidation of the primary tumor size and characteristics as well as for mediastinal lymphadenopathy or other disease burden in relation to the major structures of the thorax. Extension of CT to the liver and adrenal glands can evaluate two common sites of metastasis at this initial scan. If there is an increase in the size of lymph nodes noted on CT, positron emission tomography (PET) is useful in distinguishing malignant tissue from benign forms of lymphadenopathy. PET provides benefit in the evaluation of regional disease as well as distant sites, with the exception of visualizing disease within the brain due to baseline metabolic activity. In the PLUS multicenter randomized controlled trial, addition of PET to the preoperative workup prevented futile thoracotomy in 20% of patients with suspected NSCLC.1 Although an expensive imaging modality, the upfront cost of PET imaging has been proven to be worth it in the staging of newly diagnosed non–small cell lung cancer (NSCLC) and in the diagnosis of indeterminate solitary pulmonary nodules, as it is the most accurate non-invasive imaging modality to evaluate the mediastinum, offers additional evaluation of extrathoracic sites of possible disease, and can reduce the incidence of non-curative resections.2,3 Magnetic resonance imaging (MRI) of pulmonary or mediastinal nodules has little role to contribute at the staging period unless the patient has iodine contrast allergy. MRI is useful if the patient gives a reason for needing more detailed visualization of disease invading vascular structures, vertebral body, or the brain. Computed tomographic scanning for identification of mediastinal lymph node metastasis was found to have sensitivity and specificity of approximately 55% and 81%, respectively.3 Mediastinal lymph node staging by PET appears to have greater accuracy compared to CT, as proved in a meta-analysis with a pooled sensitivity of 84% (CI 78%-89%), specificity of 89% (CI 83%-93%), and overall prevalence of disease 32% (range 5%-56%). Combined CT and PET scan improved sensitivity to a range of 0.78-0.93 and specificity from 0.82 to 0.95, with a prevalence of mediastinal disease from 32% to 50%.4 A Cochrane review of 45 studies based on combined PET/CT positivity performed two main analyses based on PET uptake. In the first group where uptake was noted to be just greater than the background uptake, PET/CT correctly identified nodal disease metastasis beyond N1 nodes in 77.4% of patients (95% CI 65.3%-86.1%) and 90.1% (95% CI 85.3%-93.5%) of patients without disease metastasis beyond N1 nodes. In the second group where uptake was noted to have a SUVmax (maximum standard uptake value) of 2.5 or greater, PET/CT correctly identified nodal disease metastasis beyond N1 nodes in 81.3% of patients (95% CI 70.2%-88.9%) and 79.4% (95% CI 70%-86.5%) of patients without metastasis beyond the N1 nodes.5 Even with this high accuracy, it should be noted that PET analysis may lead to false negatives in disease that has low metabolic activity (eg, carcinoid and bronchioalveolar tumors). For peripherally located nodules less than 3 cm with no evidence of hilar, mediastinal, or other metastatic presentation on PET, it is indicated to forgo biopsy in favor of proceeding directly to wedge resection.6 For centrally located tumors or for tumors greater than 3 cm, invasive staging of the mediastinum is recommended even if the PET is negative, as false-negative rates have been shown to approach 20%-25%.3 Therefore, with high suspicion of malignancy, which can be aided through use of the Fleischner criteria, any imaging finding of mediastinal lymph node enlargement should be confirmed with tissue biopsy.7 Confirmation of a histologic diagnosis via direct tissue sampling also makes available molecular testing. The differentiation of a cytologic specimen is tightly associated with chemotherapy treatment regimen, and as such early assessment of tissue is imperative. Tissue can be obtained through biopsy via CT-guided needle, EBUS, bronchoscopy, mediastinoscopy, or video-assisted thorascopic surgery (VATS). Endobronchial ultrasound represents a relatively new and alternative method for nodal staging of lung cancer. In a prospective controlled trial, Yasufuku et al. found no significant differences between EBUS transbronchial needle aspiration (TBNA) and mediastinoscopy in determining the true pathologic N stage in over 150 patients.8 Further study has documented the ability to achieve high sensitivity and specificity using EBUS with rapid on-site evaluation by a cytopathologist. In a large report of 483 patients, Nakajima et al. found the sensitivity and specificity of EBUS TBNA samples to be 96.5% and 100%, respectively.9 With the ability to diagnose not only tumor type, but also molecular genotype of cancers, EBUS TBNA may replace other more invasive staging methods in patients with advanced disease.10 Those with negative results, however, should still undergo surgical staging with mediastinoscopy when there is a high suspicion for N2,3 disease.3 Mediastinoscopy involves taking a patient to the operating room. Under general anesthesia, the surgeon will make a pretracheal incision just superior to the sternal notch, dissect inferiorly along the anterior trachea to the mediastinum, and insert a mediastinoscope. Through the scope, the surgeon performs biopsies of mediastinal lymph nodes. Risks of the procedure are minimal, with one study finding morbidity and mortality of 0% and 2.1%, respectively. Complications included hoarseness, which improved at follow-up, and wound infection.11 Video mediastinoscopy uses an enhanced mediastinoscope with a camera at the distal tip of the scope blade. This technology outperforms conventional mediastinoscopy in that it yields more lymph nodes and more lymph node stations with higher accuracy, better negative predictive value, and increased sensitivity.3,12 Traditionally, the lymph nodes accessible for biopsy via mediastinoscopy are listed by station as follows: 1 (low cervical); 3a, 3p (prevascular, retrotracheal, respectively); 2R, 2L, 4R, 4L (paratracheal); and 7 (anterior subcarinal). The stations 2R, 2L, 4R, 4L, and 7 should be routinely sampled with all procedures. Video-assisted mediastinoscopic complete lymphadenectomy has been shown to have equal accuracy compared to open lymphadenectomy with the added benefit of easier access to the left paratracheal and tracheobronchial stations.13 Video mediastinoscopy is also useful in surgical training, as both attending and resident can view a screen together, as opposed to taking turns visualizing a limited field. This leads to more rapid learning without added risk to patient safety.14,15 In comparison of techniques utilized to stage mediastinal disease involvement, it is important to note that often EBUS and PET are performed on patients with enlarged mediastinal lymph nodes, with mediastinoscopy is performed on patients to rule out disease even without mediastinal lymph node enlargement.14,15 As staging of disease is confirmed, patients with stage I or II lung cancer should be considered for surgical resection. Prior to the operation, thorough history and physical examination of the patient’s overall medical status and screening with chest plain films and electrocardiography should be performed. Additionally, pulmonary function testing (PFT) should be done routinely. Those patients with signs and symptoms of cardiopulmonary disease at this initial evaluation should undergo further testing of physiological fitness for ability to tolerate an operation and reduction in lung volume. If considering pneumonectomy and patients complain of chest pain or have signs of heart failure, then echocardiography and stress testing is recommended. The pulmonary capability of patients needing treatment for lung cancer is often markedly hampered at the expense of a lifetime of abusing tobacco products. PFTs are the primary method to evaluate for operative risk, notably the forced expiratory volume in 1 second (FEV1) measured in the forced vital capacity test and the diffusing capacity of the lungs for carbon monoxide (DLCO) measured by inhalation of a test gas. We can calculate the predicted postoperative lung function with the metric ppo-FEV1 = FEV1 [1 – (Number of segments resected × 0.0526)] (where ppo refers to predicted postoperative). This equation can substitute DLCO in the place of FEV1. In the presence of significant obstruction, significant pleural disease, endobronchial obstruction, or history of prior resection, other modalities must be used to assess lung function. A variety of methods to predict postoperative functional status exists, including perfusion scans and quantitative CT. The correlations between pre- and postoperative functional status proved perfusion and CT scanning were useful predictors regardless of the extent of resection, with perfusion results being the most accurate. Predictions based on counting functional anatomic segments found on imaging were less accurate and could only be found useful for resections limited to one lobe.18 A quantitative or ventilation-perfusion lung scan is helpful in predicting the postoperative function in marginal cases where FEV1 and DLCO are less than 60%-80%. If ppo-FEV1 and ppo-DLVO are greater than 40% on this scan, there is an acceptable risk in attempting surgery.19 If less than 60% but greater than 30% predicted, patients may be evaluated with a low-technology exercise test such as stair climb or shuttle walk test. If less than 30%, then a formal cardiopulmonary exercise test (CPET) with measurement of maximal oxygen consumption (VO2max) is recommended. The Thoracic Revised Cardiac Risk Index (ThRCRI) is a branch of the Revised Cardiac Risk Index (RCRI) used to specifically evaluate patients for cardiac risk prior to lung resection. It can be used as a screening tool to separate those individuals who need further cardiac assessment from those who are fit for surgery. Of classes A (score 0-1), B (1.5-2.5), and C (>2.5), those within the class C category had a shorter 5-year overall survival (OS), shorter cancer-specific survival (CSS), and higher mortality from specifically cardiac events.20,21 In patients with need of further cardiac assessment, exercise testing, echocardiography, or nuclear perfusion scans should be performed and any reversible pathology addressed prior to initiation of therapy for the cancer. Cardiopulmonary exercise test is considered the gold standard for preoperative risk assessment. It includes recording electrocardiogram, heart rate, minute ventilation, and oxygen uptake per minute during exercise. The maximal oxygen consumption (VO2max) is measured from this test. It has been studied extensively regarding consideration for preoperative risk stratification and found to be an independent predictor of postoperative pulmonary morbidity and mortality. Patients with VO2max greater than 20 mL/kg/min can safely undergo resection up to pneumonectomy, as morbidity rates have been observed at 3.5% with no deaths. In patients with VO2max less than 12 mL/kg/min, the morbidity and mortality rates were significantly higher at 33% and 13%, respectively.22 If VO2max is less than or equal to 10 mL/kg/min, surgery is contraindicated, and patients should be counseled on non-operative management of their lung cancer. In general, regarding the fitness of a patient under consideration for surgical resection, there is acceptable risk if ppo-DLCO and ppo-FEV1 are greater than 40% and VO2max is greater than 15. High-risk patients are those with ppo-DLCO and ppo-FEV1 within 20%-40% and VO2max 10-15. With ppo-DLCO and ppo-FEV1 less than 20% or VO2max < 10, surgery is not indicated.23 Smoking is the foremost cause of preventable death.24 While the proportion of adult daily smokers in the United States has declined in recent years, it continues to have a prevalence of 17.5% of men and 13.5% of women.25,26 Recent reports have indicated that 66.7% of men and 69.4% of women expressed an interest in smoking cessation; however, less than half used counseling and/or medication in their efforts.27 Overall, cancer patients and cancer survivors who smoke have increased all-cause mortality, cancer-specific mortality, and risk for a second primary cancer.24 In their study of lung cancer patients, Dobson Amato et al. found a median 9-month improvement in OS in patients who quit tobacco after diagnosis.28 Specific to thoracic surgery, the association between smoking and adverse postoperative outcomes is well known.29–31 Even as more minimally invasive procedures are utilized, smoking remains a risk factor for complications. In a prospective study of lung cancer patients undergoing VATS, multivariate analysis revealed current smoking as a significant independent risk factor for postoperative pulmonary complications.32 The American College of Chest Physicians recommends that all patients with lung cancer who are being considered for surgery and who are actively smoking be treated for tobacco dependence.33 The 2013 guidelines recommend perioperative cessation with pharmacotherapy. If patients have contraindications to or refusal of pharmacotherapy, cessation counseling alone is recommended. Importantly, the authors of this chapter do not advocate for the delay of surgical procedures in favor of a longer period of abstinence, as the timing of cessation does not appear to increase the risk of postoperative complications.34 The National Comprehensive Cancer Network has outlined concordant principles of smoking cessation, enforcing that surgical patients should be encouraged to quit smoking, emphasizing again that longer periods of abstinence should not delay the planned surgical procedure.35 In light of this evidence, a recent survey of thoracic surgeons demonstrated significant variability in the management of smoking patients related to denying procedures to smokers, duration of preoperative abstinence from smoking, and nicotine testing prior to surgery.36 When instituting a smoking cessation regimen to a patient’s care, multiple evidence-based methods are available. These included pharmacotherapy and behavioral therapy. Several randomized studies have shown the superiority of varenicline to both placebo and bupropion in patients who want to quit smoking.37,38 Moreover, in patients not ready to quit smoking but willing to reduce, varenicline also demonstrated significantly better abstinence rates in a randomized study against a placebo.39 The incorporation of the “five major steps to intervention” (ask, advise, assess, assist, arrange) allows the surgeon to document the smoking history, introduce smoking cessation and its benefits, evaluate the patient’s readiness to quit, assist with pharmacotherapy initiation, and arrange follow-up to monitor progress toward smoking cessation.1 This strategy can be assimilated into clinic workflow with the assistance of other personnel, making it possible for the physician to spend less than 60 s fulfilling this vital part of the preoperative visit.35 The exercise capacity of patients undergoing lung resection, expressed as VO2max, has been proven to be lower in those patients who develop pulmonary complications and inversely proportional to length of stay postoperatively. It has also been shown that preoperative pulmonary rehabilitation may lead to improvement in VO2max.40–42 One study testing pulmonary rehabilitation in patients with chronic obstructive pulmonary disease (COPD) noted a shorter hospital stay by 3 days, fewer prolonged chest tubes (defined as >7 days), and fewer days needing a chest tube compared to controls with 10 preoperative sessions involving inspiratory muscle training, endurance training, and practice of slow breathing.43 The benefit of preoperative exercise training with the idea of improving a patient’s postoperative recovery seems to fit logically but has proven difficult to quantify. Studies regarding preoperative physiotherapy effects on reduction of postoperative pulmonary complications thus far have been limited in power and statistical significance but do warrant further exploration with large, multicenter, randomized controlled trials. A 58-year-old gentleman presents to your office after a 1.1-cm right upper lobe nodule was found incidentally while undergoing CT examination workup after a car crash 1 month prior. He currently denies any shortness of breath, hematemesis, cough, or recent bought of pneumonia. He has a 20 pack-year history of cigarette smoking, yet he stopped smoking 18 years ago. He denies any past medical history or family history. Learning Objectives: 1. What are the modalities for staging lung cancer? 2. What is the ideal treatment approach for resectable non-metastatic early stage lung cancer? 3. Which lymph nodes are sampled with a mediastinoscopy? 4. Should the patient have a robotic or video-assisted surgery? Treatment for patients with stage I and II cancer are reviewed in this chapter. Clinical staging does not always correlate with pathological staging, leading to most patients being restaged after surgical sampling of mediastinal nodes. For example, 28% of patients with clinical stage I lung cancer were upstaged (14% stage II and 14% stage III) in a Cancer and Leukemia Group B prospective clinical trial (CALGB 9761).44 The mediastinum is sampled either at the time of resection or prior to resection, depending on the suspicion of the physician on the presentation of the patient (Figure 13-1). Figure 13-1. Standard mediastinal scope insertion technique used in a mediastinoscopy for nodal staging. (Reproduced with permission from E Hong, MJ Liptay. Techniques for staging and restaging of lung cancer. In Sugarbaker DJ, Bueno R, Colson YL, et al., eds. Adult Chest Surgery. 2nd ed. McGraw Hill Medical; 2015: Chap. 70. Available at https://accesssurgery.mhmedical.com/content.aspx?bookid=1317§ionid=72431939. Accessed June 10, 2019.) Surgical resection presents the greatest opportunity for survival when patients present with early stage I or II NSCLC.45,46 Although a mass may be amenable to surgical resection, it does not mean that a patient is a surgical candidate for the operation at hand. It is essential that all patients undergoing lung resection receive preoperative evaluation. Preoperative evaluation is discussed in detail in a separate chapter. For patients who are considered surgical candidates, an R0 surgical resection is the procedure of choice for patients who present with stage I and II disease. For patients who undergo resection and are unable to obtain a negative margin, R1 resection, there is a role for postoperative chemotherapy with radiation. Lobectomy continues to be the procedure of choice for resection in early stage NSCLC.47 Traditionally, lobectomy was performed with an open thoracotomy technique, yet as technology has advanced, more studies continue to advocate for a minimally invasive surgical approach.48 The options for minimally invasive approaches include both the traditional VATS and newer robotic-assisted thoracic surgery (RATS), which is starting to gain favor among thoracic surgeons. Bilobectomy is the removal of two lobes on the right side. Traditionally, it has been considered a high-risk procedure because it has the potential for significant morbidity and mortality with the addition of possible negative impact on OS.49 Galetta et al. recently presented a retrospective review that had favorable survival rates based on stage when compared to lobectomy, with high morbidity but low mortality rates when performing bilobectomy.49 Although not commonly performed and with high morbidity rates, bilobectomy appears to be an acceptable alternative operation when compared to lobectomy when criteria for bilobectomy are met but lobectomy would not be acceptable for oncologic reasons. Although lobectomy might be considered the “gold standard” for peripheral tumors, as tumors become more proximal, a lobectomy may not be adequate for R0 resection. Such tumors can be approached with a pneumonectomy or a tracheobronchial sleeve resection. Pneumonectomy is an operative procedure in which the entirety of the lung is removed at the time of surgical resection. To undergo this operation, a patient has to have enough pulmonary reserve to tolerate the operation. Even with the significant advancements made in the field of thoracic surgery since the first reported successful 1-stage pneumonectomy performed in 1933 by Graham and Singer, pneumonectomy still has a high complication rate.50 Due to the pulmonary insult a patient undergoes after pneumonectomy, this has led to the development and advancement of the tracheobronchial sleeve resection, commonly referred to in short as a sleeve resection. A sleeve resection is circumferential resection of the involved airway with primary end-to-end anastomosis. Pneumonectomy is fraught with postpneumonectomy complications that not only involve the respiratory system of the patient but also can encompass cardiovascular and pleural space disease.51 Due to the extent of morbidity that is associated with the postpneumonectomy state, a sleeve resection is generally favored.52 Sleeve resections have been shown to have similar oncologic outcomes when compared to pneumonectomy. Furthermore, they allow for greater preservation of pulmonary function, have better outcomes with long-term survival, increase quality of life, and are overall more cost-effective.52 With the advancement in surgical technique since its inception, tracheobronchial sleeve resection has established itself as the preferred resection for the majority of centrally located tumors. Sublobar resection (SR) can be classified as either a non-anatomical wedge resection or an anatomical segmentectomy. Both of these resections allow patients who might not tolerate a formal lobectomy due to poor pulmonary status to undergo resection when the tumor is amenable to this type of resection. The initial data reported by the Lung Cancer Study Group Trial 801 demonstrated an increased rate of local recurrence and lower survival rates when comparing limited resection to lobectomy.47 This study’s application to current practice, however, has its impediments, as it was reported over 20 years ago, without the use of PET imaging and included wedge resection with segmentectomy in 1 analysis.47 Another more recent series by Dai et al. investigated 15,760 patients with T1aN0M0 NSCLC tumors (≤2 cm, now T1a and T1b tumors) from the SEER (Surveillance, Epidemiology, and End Results) database. OS and lung cancer-specific survival (LCSS) were evaluated in patients comparing outcomes after lobectomy, anatomical segmentectomy, or non-anatomical wedge resection.53 They showed that lobectomy had a greater survival advantage for patients with NSCLC ≤ 1 cm and 1 to 2 cm. OS and LCSS for tumors greater than 1 to 2 cm were lower after wedge resection compared to lobectomy. Yet, for patients who had a wedge resection for NSCLC tumors ≤ 1 cm, survival was similar.53 Cardinale et al. reported another favorable database review comparing segmentectomy to lobectomy from 1998 to 2006 in the SEER database for early stage Ia NSCLC.54 Their results favored segmentectomy with significant overall and LCSS when compared to wedge resection.54 As surgeons become more familiar with minimally invasive techniques and advancement in preoperative screening techniques detect smaller lung nodules, long-term outcomes can be evaluated on patients who receive SRs. Newer reports have had favorable results in favor of segmentectomy. Recently, a large retrospective study investigating the Poland National Lung Cancer Registry failed to show a statistical difference in 3-year and 5-year survival rates when comparing lobectomy to segmentectomy.55 This survival advantage was not observed when comparing wedge resection to lobectomy, noting that 3- and 5-year survival was statistically less for patients who underwent wedge resection.55 Although more recent study results are starting to show segmentectomy as an oncologic equivalent in terms of survival to a lobectomy, the overall consensus appears to still be up for debate. It would be safe to say that a lobectomy can still be considered the gold standard for early stage NSCLC. Yet, for a patient who might not tolerate a complete lobe resection, a segmentectomy is a possible option for a patient with predicted borderline pulmonary function after a complete lobectomy. The majority of lobectomies are still performed utilizing the open approach, despite an increasing amount of data that show the benefit of minimally invasive approach.56 VATS offers a minimally invasive approach to the traditional open thoracotomy technique for early stage lung resections. Commonly accepted incision placements for VATS operative technique are shown in Figure 13-2. A typical operating room setup during VATS is pictured in Figure 13-3, with operating room bedside port placement shown in Figure 13-4. Figure 13-2. Commonly accepted incision proposal for port placement in a video-assisted thoracoscopic surgery. (Reproduced with permission from R. J. McKenna. Atlas of Minimally Invasive Thoracic Surgery (VATS). Elsevier; January 1, 2011:3-14, Figure 1-2, Incisions for a video-assisted lobectomy. Copyright © 2011 by Saunders, an imprint of Elsevier Inc.) Figure 13-3. A common operating room setup during a video-assisted thoracoscopic surgery. (Reproduced with permission from Thirugnanam A. Video-assisted thoracoscopic surgery and open chest surgery in infectious lung diseases. J Vis Surg. 2017;3:3. Published 2017 Jan 6. doi:10.21037/jovs.2016.12.03.) Figure 13-4. Picture of bedside placement of ports and trocars during a video-assisted thoracoscopic surgery. (Reproduced with permission from Thirugnanam A. Video-assisted thoracoscopic surgery and open chest surgery in infectious lung diseases. J Vis Surg. 2017;3:3. Published 2017 Jan 6. doi:10.21037/jovs.2016.12.03.) In a prospective trial of 128 patients with peripheral lung nodules less than or equal to 3 cm, a standardized VATS technique was shown to have acceptable outcomes.57 VATS resection has been reported to reduce the length of stay without compromising oncologic outcomes when compared to an open technique.48 Most recently, VATS lobectomy was shown to have overall lower 30-day morbidity when compared to the open approach.59 With the advancements of minimally invasive surgery and technology, RATS is starting to build steam as another acceptable alternative to open thoracotomy for early stage NSCLC. Port placement and a docked Xi robot are shown in Figure 13-5. Figure 13-5. A. Trocar port placement for robotic-assisted thoracoscopy surgery right upper lobectomy. B. Xi robot docked in operative position. (Reproduced with permission from Kim MP, Chan EY. “Five on a dice” port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J Thorac Dis. 2017;9(12):5355–5362. doi:10.21037/jtd.2017.11.0.) At the onset of RATS, early reports of RATS lobectomy appeared to have consistent and favorable outcomes when compared to the current standards of VATS lobectomy.60 As this operation has developed over the years latter reports, including a large multi-institutional retrospective review of robotic lobectomy during an 8 year period showed low morbidity and mortality with acceptable long term survival that was similar to VATS and open resection.61 In addition, a more recent study retrospectively reviewed 10 years of robotic resection at their institution for stage I and II NSCLC and had favorable results.62 They had excellent 30- and 90-day mortality, 0% and 0.3%, respectively.62 In addition the oncologic survival at 3 and 5 years was similar to results seen from VATS and open technique, at 96.1% and 91.5%, respectively.62 Both of these studies are encouraging for the future of robotic-assisted resection in terms of oncologic outcomes and low morbidity and mortality from the operation itself. The technology continues to become more readily available for the thoracic surgeon to utilize in their practice. Since the majority of hospitals will not have a robot at their disposal, proficiency in open and VATS surgical approach is still necessary. Although most early stage resections that undergo an open, VATS, or RATS approach will not need postoperative intensive care, its availability is imperative in case of intraoperative and postoperative complications. Favorable data from advance recovery protocols seen from early implementation in elective colorectal surgery has led the field of thoracic surgery to investigate protocols for enhanced recovery protocols (ERPs) in thoracic resections.63–65 Recent outcomes investigating results from implementation after ERP have been favorable but have not been widely adopted.66 As advances continue to occur in diagnostic and therapeutic interventions in NSCLC, the treatment algorithm will continue to evolve and improve. The surgical field continues to shift based on the data derived from prospective and retrospective studies. As data continue to support the use of the VATS and now RATS operative technique in terms of overall and oncologic survival outcomes, faster patient recovery, and overall patient satisfaction, these technologies are still not readily available to all surgeons but appear to be taking a strong hold on the future of operative intervention for early stage NSCLC. A 60–year-old Asian female had a 4-cm lung mass biopsied by core needle biopsy. She never smoked and has no history of cancer or comorbidities. The pathology shows adenocarcinoma of the lung. PET and MRI are otherwise negative. Learning Objectives: 1. Who would be a candidate for curative treatment of early stage lung cancer by radiation? 2. What are the risks of stereotactic radiation? 3. How long does the radiation treatment take? Patients with early stage NSCLC are managed with surgical resection or definitive radiation therapy (RT). Surgical considerations are discussed elsewhere, but, to briefly summarize, lobectomy is considered the standard of care for medically fit patients capable of tolerating the procedure.67 Many patients in this population have long smoking histories, which carry associated pulmonary and cardiovascular medical comorbidities, increasing their operative risk. Patients deemed to be at a high operative risk are not candidates for lobectomy, at which point their options are less extensive surgery or non-operative management. While segmentectomy or wedge resection offers the ability to remove tumors with a smaller concomitant reduction of healthy lung parenchyma, these procedures are less ideal and not considered an oncologic surgery, as normal tissue sparing comes with the compromise of a greater incidence of local recurrence.47 Determination of operability is guided by factors such as advanced age, cardiovascular or pulmonary impairment, and the burden of competing comorbidities. Objective quantification has mainly relied on PFT, specifically the FEV1 and the DLCO. High operative risk and medically inoperable patients are candidates for definitive RT after multidisciplinary discussion has concluded the morbidity of surgical resection outweighs potential benefit. Surgical resection is recommended for patients deemed medically fit to undergo the procedure, with evidence-based guidelines available to aid this decision. 67 For these patients, RT is generally reserved for the postoperative setting when lymph node sampling demonstrates metastatic carcinoma, diagnosing the patient with locally advanced disease.68 Multiple studies have sought to determine a role for postoperative radiation therapy (PORT) in early stage lung cancer, but results were mixed, with no clear improvement in survival. Ultimately, a meta-analysis of these trials demonstrated PORT to be detrimental in stage I-II patients.69 A subsequent SEER database analysis further showed a negative impact on survival, with benefit seen only for locally advanced patients with N2 disease.70 For patients with positive margins on resection (R2), PORT improves survival, and this effect is seen for all nodal stages.71 For patients deemed high risk for lobectomy, SR provides a less morbid operation, but with a higher rate of local recurrence. In the American College of Surgeons Oncology Group (ACOSOG) Z4032 trial, investigators sought to improve local control (LC) with the addition of postoperative brachytherapy. Patients with stage I NSCLC meeting criteria warranting exclusion of lobectomy underwent SR and were randomized to observation or brachytherapy. The brachytherapy group underwent iodine 125 suture or mesh implant at the postresection staple line at time of SR. Unfortunately, final analysis demonstrated no statistical difference in local progression; however, on subset analysis there was a trend toward significance for a benefit with brachytherapy in patients with positive staple line cytology.72 Historically, primary RT was typically reserved for medically inoperable patients as the only available option for definitive treatment. While conventional RT increased survival compared to no treatment, the results were lackluster, with a survival benefit of 5-7 months and no change in 5-year OS.73 Over the past few decades, as medical radiation technology improved, so did the treatments and results. Improved outcomes were seen when 3-dimensional RT was compared to older 2-dimensional techniques.74 A retrospective series from Memorial Sloan Kettering Cancer Center (MSKCC) showed that for patients with stage I/II tumors, doses of 80 Gy or greater using 3-dimensional conformal RT with sequential chemotherapy yielded a median OS of 3.4 years.75 An ability to better deliver a dose led to dose escalation studies demonstrating these treatments to be safe with acceptable toxicity, including when administered with chemotherapy.76,77 To achieve these higher doses, conventional daily 2-Gy fractions required treatment periods of 6+ weeks, which can be very demanding on patients, especially those with significant comorbidities that have precluded surgery. Further work showed accelerating treatments with hypofractionated regimens to be feasible, reducing treatment time by up to 2-3 weeks with similar rates of control and low toxicity.78 Stereotactic body radiation therapy (SBRT) has emerged as the standard of care for medically inoperable patients with early stage NSCLC. This technique utilizes patient immobilization, image guidance, and respiratory management to deliver very high doses of radiation in a much shorter course than previous conventional regimens. Early retrospective comparisons between SBRT and conventional RT demonstrated improved local control (LC) and OS in favor of SBRT, with a meta-analysis estimating an improvement in 5-year OS from 19% to 42% when comparing conventional RT to SBRT.79,80 Since the mid-2000s, multiple fractionation schemes have been studied in single- and multiple-institution phase 2 studies with excellent results, reporting LC rates of more than 90% and 3-year OS rates of 55%-60% in stage I patients.81,82 Of note, this treatment is also referred to as stereotactic ablative radiation therapy (SABR), but this nomenclature is not used in this chapter. By increasing the dose of radiation delivered per treatment fraction, radiation oncologists are able to take advantage of radiobiological properties and achieve a higher biologically effective dose (BED). A higher dose delivered to a tumor necessarily results in a higher dose to surrounding normal tissue, and for these treatments to be feasible, the amount of normal tissue receiving a high dose must be minimized. The stereotactic approach involves creating a treatment setup and plan that maximally minimizes aspects of uncertainty in tumor location. The first step is creating reliable and reproducible patient positioning and may be accomplished with rigid immobilization techniques to ensure the same area of the body is targeted with each treatment with minimal change in the patient’s body position in space (Figure 13-6). Figure 13-6. Patient setup for stereotactic body radiation therapy for treatment of a stage I lung cancer. A vacuum lock bag is used for rigid immobilization with plastic wrap over the top for further immobilization. Motion management is being used, with a block on the chest to measure surface position change with breathing, and a goggle screen gives the patient visual representation of this motion to help coordinate breathing actions. Because of the movement of the chest and intrathoracic contents with respiration, the next step involves accounting for the position of the tumor in space and time. Multiple methods have been developed to accomplish this. Four-dimensional CT (4-D CT) simulations are performed for treatment planning, allowing tumor motion to be tracked through all phases of the breathing cycle. Qualitative and quantitative vector maps have been used to describe lung and tumor motion that must be accounted for during RT delivery. Lung tumors can move well over 2 cm depending on the tumor location within the lung (Figure 13-7).83 Figure 13-7. MRI depiction of parenchymal lung motion. A. Patient with solitary non–small cell lung cancer in upper right lung. B. Using simple vector field representation for a one-time frame of the breathing cycle, limited lung motion in right upper lung can hardly be seen. C. Using color map representation of the breathing cycle, from maximum expiration to maximum inspiration, limited asymmetric lung motion can clearly be seen. In this patient, overall lung motion was limited because of limited lung function. (Reproduced with permission from Plathow C, Schoebinger M, Herth F, Tuengerthal S, Meinzer HP, Kauczor HU. Estimation of pulmonary motion in healthy subjects and patients with intrathoracic tumors using 3D-dynamic MRI: initial results. Korean J Radiol. 2009;10(6):559-567.) Using this information, the treating radiation oncologist can employ multiple techniques to ensure accurate targeting. For tumors that move minimally, a small extra margin can be applied to cover the target in all phases of the breathing cycle (Figure 13-8).84 If there is substantial motion, radiation can be delivered in a gated fashion, with dose only being delivered at certain intervals of the breathing cycle as tracked by external monitoring or by having the patient hold his or her breath during the periods of radiation delivery to “fix” the tumor in space by eliminating intrathoracic respiratory motion (Figure 13-9).85 Figure 13-8. Example of internal target volume (ITV) creation. CT imaging is obtained for all phases of the breathing cycle, and the tumor is contoured in each phase to create a target volume that encompasses all areas it occupies. (Reproduced with permission from Glide-Hurst C, Chetty I. Improving radiotherapy planning, delivery accuracy, and normal tissue sparing using cutting edge technologies. J Thorac Dis. 2014;6(4):303-318. http://jtd.amegroups.com/article/view/2119.) Figure 13-9. Principle of respiratory gating. The tumor is tracked during all stages of the respiratory cycle, and a particular phase is chosen for treatment. The radiation beam is turned on, and treatment is delivered during this prespecified phase and is turned off at other times so that the beam targets the tumor at a particular point in time and space and avoids treatment of normal lung parenchyma when the tumor moves during the next phase of breathing. (Reproduced with permission from Kim JH. LINAC-based high-precision radiotherapy: radiosurgery, image-guided radiotherapy, and respiratory-gated radiotherapy. J Korean Med Assoc. 2008 Jul;51(7):612-618. https://doi.org/10.5124/jkma.2008.51.7.612.) Pretreatment imaging is mandatory for SBRT to ensure accurate treatment localization prior to radiation delivery, but intratreatment radiographic imaging can also be used to track the tumor, sometimes with the aid of fiducial markers or by confirmation of the positioning of adjacent bony structures (Figure 13-10). There is growing interest in using implanted fiducial markers with positional tracking radio transmission capabilities to track tumor motion, and this will be an area of active study in the coming years (Figure 13-11). Figure 13-10. Fiducial markers. Metallic fiducial markers were bronchoscopically placed into a left upper lobe lung tumor and are utilized by the treatment delivery system to track the tumor throughout the breathing cycle to aid in targeting. Figure 13-11. Workflow. This diagram details the steps for SBRT treatment of a lung tumor, from the initial planning stage to the delivery of treatment. (Reproduced with permission from Abreu CECV, Ferreira PPR, Moraes FY, Neves WFP Jr, Gadia R, Carvalho H A. Stereotactic body radiotherapy in lung cancer: an update. J Bras Pneumol. 2015;41(4):376-387. https://dx.doi.org/10.1590/S1806-37132015000000034.) Stereotactic radiosurgery (SRS) is the use of high-dose RT, which is by definition delivered in a single fraction, to intracranial neoplasms, commonly in the form of brain metastases. As this technology gained widespread acceptance in the late twentieth century as an alternative to surgery, groups in Japan and the United States began exploring the feasibility of applying this technique to small lung tumors. First described as extracranial SRS, this technique then became known as SBRT to refer to RT delivered in a stereotactic manner to the body. Uematsu et al. retrospectively analyzed a group of patients treated in the late 1990s undergoing RT to small primary lung tumors and lung metastases using motion evaluation, frame immobilization, and high dose per fraction (30-75 Gy delivered in 5-15 fractions). They found this technique was technically feasible, efficacious, and tolerable and yielded excellent LC.86 This finding lead to implementation of SBRT for the treatment of stage I NSCLC in Japan; however, uncertainty remained regarding optimal dosing. The Japanese Society of Radiation Oncology (JCOG) analyzed multiple fractionation regimens in both operable and inoperable patients receiving hypofractionated stereotactic radiation and found that doses with a BED of 100 Gy or greater provided better LC and OS.87 Updated results demonstrated a persistent benefit at 5 years, with local failure of 43% versus 8% and 5-year OS of 31% versus 71% in tumors dosed to a BED less than 100 Gy versus BED greater than 100 Gy, respectively.79 While these previous findings were in a cohort receiving multiple fractionation regimens, Nagata et al. analyzed a population who had received 48 Gy in 4 fractions in a phase 1/2 study. In this retrospective study, no grade 3 toxicity was reported, and at 3-year disease-free survival (DFS) was greater than 70%, and OS was 83% and 72% for stage IA and IB (<4-cm tumor size) lung cancers, respectively.88 This led to a prospective clinical trial, JCOG 0403, evaluating this dose in both medically inoperable patients and patients who were operative candidates but had declined surgery. Grade 3 and 4 toxicity was observed in this study, likely owing to the more rigorous data collection inherent to a clinical trial, in 10 and 2 patients, respectively, out of a cohort of 169. Excellent LC and OS were once again demonstrated, with 3-year OS of 59.9% and 76.5% in the medically inoperable and operable groups, respectively, and due to the low incidence of severe toxicity, this regimen became the standard of care in Japan for medically inoperable patients with stage I NSCLC.89 Meanwhile, in the United States, a group at Indiana University led by Timmerman conducted a prospective phase 1 trial evaluating dose escalation in medically inoperable stage I NSCLC patients. Escalation was conducted separately between stage IA and IB patients, with both groups reaching a dose of 60 Gy in 3 fractions without reaching a maximum tolerated dose (MTD). Of note, 2 patients developed grade 3 toxicity; however, both received dose schedules less than the maximum of 20 Gy per fraction.90 This was followed by a prospective phase 2 trial of medically inoperable patients. Patients were stratified by tumor size, with T1 patients receiving 60 Gy in 3 fractions and, as the MTD had not been reached on the phase 2 study, T2 patients receiving 66 Gy in 3 fractions. The 3-year LC was 88%, and CSS was 83%, with failures documented in the regional nodes in 9% and distantly in 13%.91 The success of this work led to the multi-institutional cooperative study Radiation Therapy Oncology Group (RTOG) 0236, a phase 2 trial delivering a dose of 54 Gy in 3 fractions to peripheral T1-2 tumors in non-operable patients, which further showed excellent disease response, with 98% primary tumor control and 91% LC (tumor and involved lobe) and a median OS of 4 years.82 The recently issued final report demonstrated further recurrences as a consequence of longer follow-up, but outcomes were promising compared to historical data, with 5-year DFS and OS of 25.5% and 40%, respectively. At 5 years, primary tumor failure was 7.3%, with lobar, locoregional, and disseminated failures reported as 20%, 25.5%, and 23.6%, respectively. Disseminated disease was more common in T2 tumors, with 45.5% versus 18.2% incidence for T1 and non-squamous histologies, with 31.6% versus 5.9% incidence in those with squamous histology, following with our understanding of the course of larger and more biologically aggressive tumors.92 Stereotactic body RT has provided major gains in survival and control in medically inoperable patients, and these results are also seen in the elderly population, for whom surgery, while feasible, comes with an increased risk of morbidity and mortality due to coexisting medical issues (Table 13-1).93 With such promising results, the question has been posited regarding whether SBRT could be used as an alternative to surgical resection in the medically operable. This is a topic of multiple current clinical trials and is discussed further in the chapter. This section reviews the results of SBRT in the medically operable population. TABLE 13-1 Radiation Dose Fractionation Schemes
13
NON–SMALL CELL LUNG CANCER TREATMENT: EARLY STAGE
PREOPERATIVE WORKUP FOR EARLY STAGE NON–SMALL CELL LUNG CANCER
HISTORY AND PHYSICAL
ROLE OF CONTRASTED COMPUTED TOMOGRAPHY AND POSITRON EMISSION TOMOGRAPHY
ENDOSCOPIC BRONCHIAL ULTRASOUND
MEDIASTINOSCOPY
DETERMINING OPERABILITY
Cardiopulmonary Testing
Pulmonary Function Tests
Cardiac Evaluation
Exercise Tolerance (VO2max)
OPERATIVE PREPARATION
Cessation of Smoking
Preoperative Rehabilitation
SURGERY FOR EARLY STAGE NON–SMALL CELL LUNG CANCER
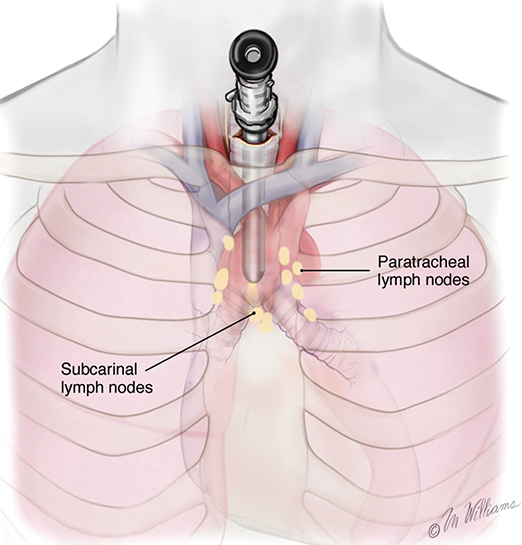
LOBECTOMY, BILOBECTOMY, PNEUMONECTOMY, AND SLEEVE RESECTIONS
SUBLOBAR RESECTION
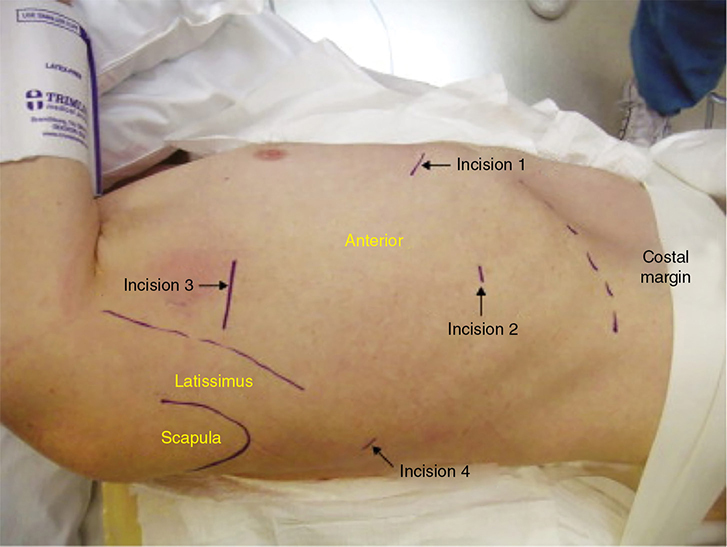
Video-Assisted Thoracoscopic Surgery
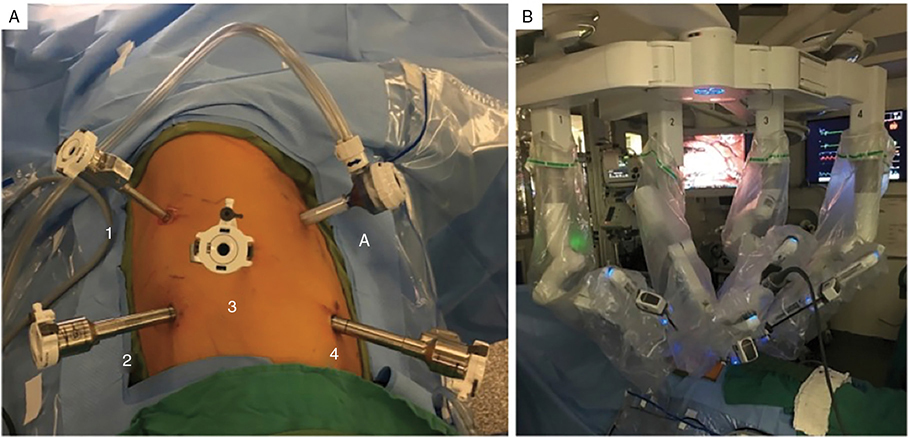
Robotic-Assisted Thoracoscopic Surgery
Postoperative Considerations
RADIATION THERAPY FOR EARLY STAGE NON–SMALL LUNG CARCINOMA
PATIENT SELECTION FOR PRIMARY THERAPY
PRIMARY SURGERY: POSTOPERATIVE RT
PRIMARY RADIATION
Stereotactic Body Radiation Therapy
Treatment Technique
Medically Inoperable Patients
Medically Operable
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree