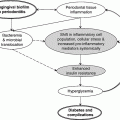
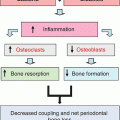
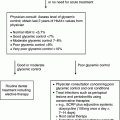
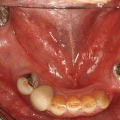
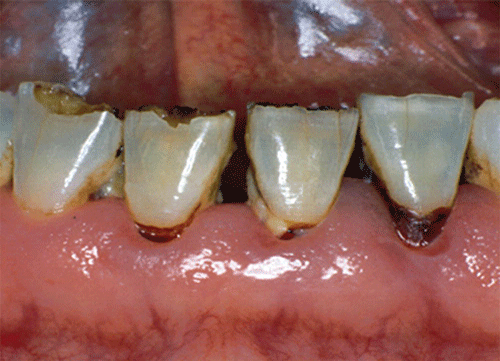
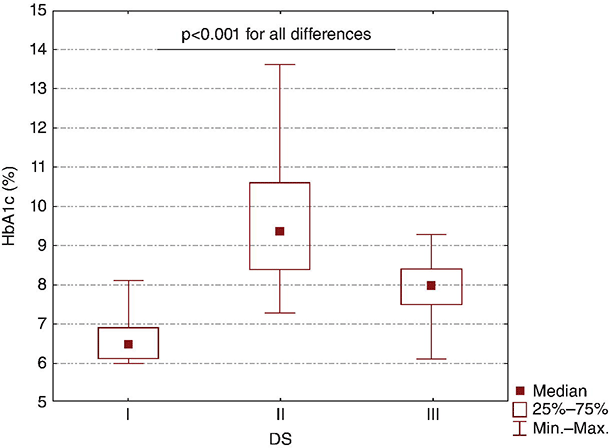
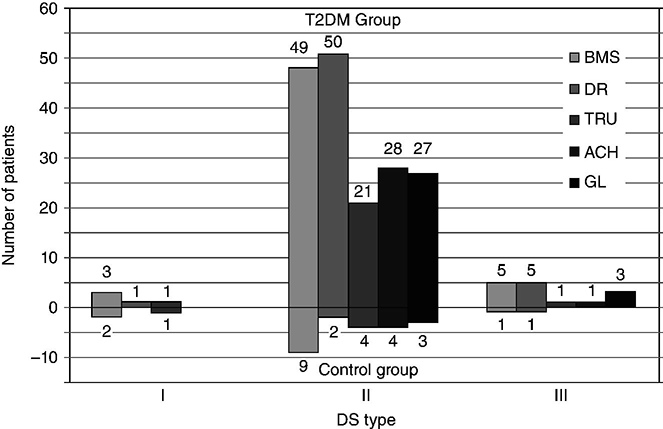
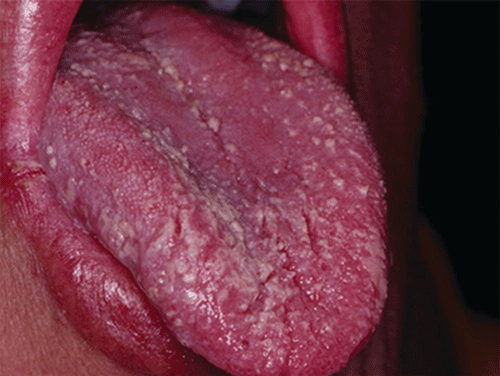
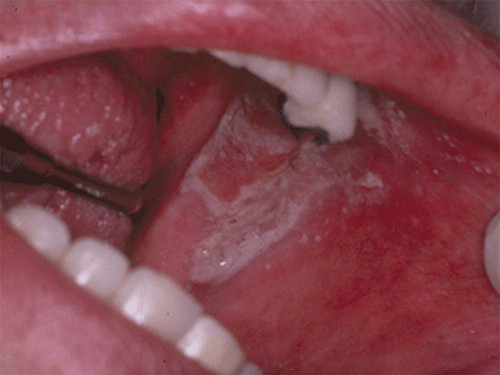
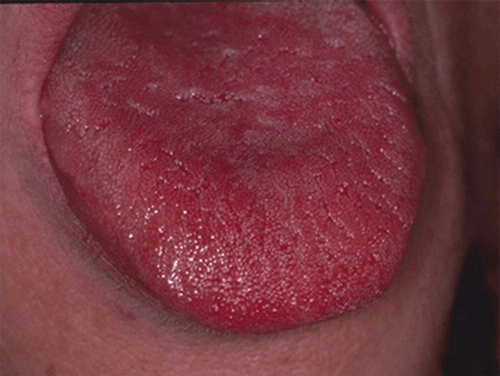
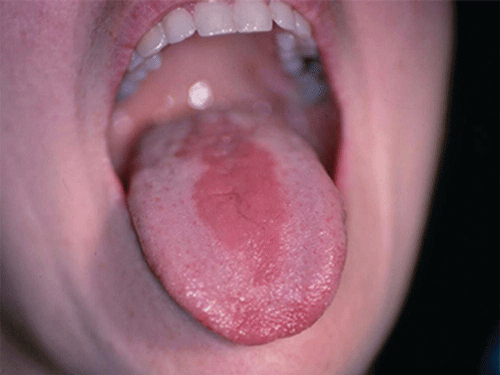
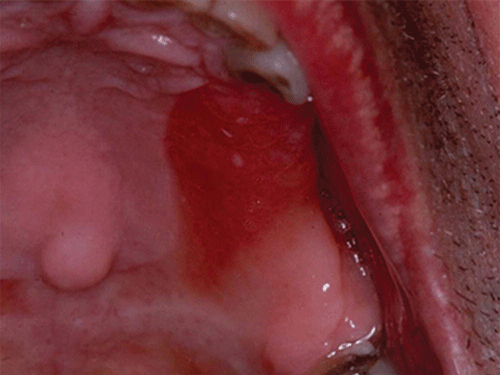
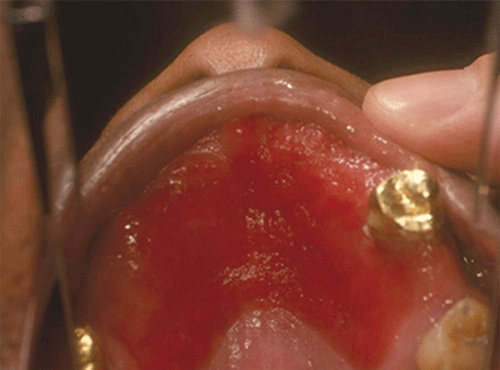
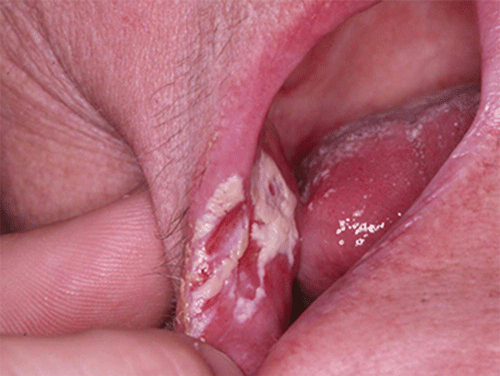
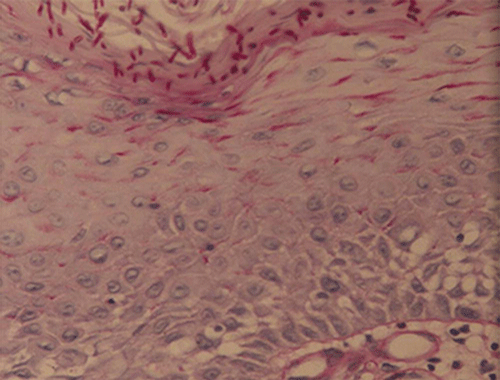
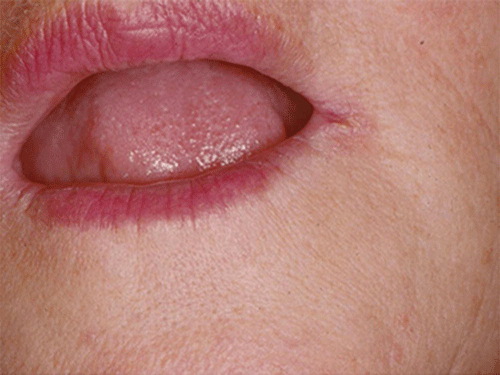
Chapter 8 Ira B. Lamster, DDS, MMSc Periodontal disease is the most important oral complication of diabetes mellitus. Not only is the severity of periodontal disease greater in patients with diabetes (Chapter 6), but periodontal disease can adversely affect metabolic control in patients with the disease (Chapter 7). However, a number of other oral lesions and disorders have been shown to occur in patients with diabetes. The prevalence of diabetes, and this spectrum of oral pathology, suggests that diabetes mellitus is the most important systemic disease encountered in patients presenting to the dental office. Dental caries is the result of acid demineralization of the teeth. Four components are required for dental caries to develop: a susceptible host (the tooth surface), specific bacteria that colonize the host (Streptococcus mutans, Lactobacillus species), a substrate available for metabolism (fermentable carbohydrates in the diet), and time. The metabolism of carbohydrates by bacteria yields lactic acid as a byproduct. Lactic acid acts on the tooth surface to cause demineralization. Time is important because the frequency of acid exposure is related to demineralization of the tooth surface. Dental caries can be broadly defined as coronal caries and root caries. Coronal caries affects the crown portion of the teeth, which is covered by enamel, and is the part of the tooth generally visible in the oral cavity. Enamel is inert. Below the enamel is the dentin, which contains cell process from odontoblasts that line the pulp chamber. Dentin has the capacity to repair. Root caries affects the roots of teeth, which in the completely healthy state are not exposed to the oral cavity. A root surface is exposed when the coronal aspect of the gingiva (and the underlying bone) is lost as a result of trauma (such as toothbrush abrasion), anatomical factors (if the teeth are positioned towards the buccal, away from the central portion of the alveolar bone), and periodontal disease (gingival recession occurs when periodontitis is present). As a result of the inflammatory process that characterizes periodontitis, both mineralized and non-mineralized supporting tissues for the dentation are lost, and the tooth appears longer in the mouth. The root surface is covered with a thin layer of mineralized tissue known as cementum. Cementum is more susceptible than enamel to the effects of lactic acid. Coronal dental caries is an extremely common disorder of childhood, and affects both the primary and permanent dentitions. Root caries is a lesion seen in adults. The effect of diabetes mellitus on the prevalence of dental caries has been widely studied. This assessment is complicated by the overlapping effects of lifestyle and metabolic management, as well as other complications of diabetes on oral health. For dental caries, potential influences include diet, salivary flow, the effect of medications, availability of susceptible tooth surfaces, the frequency with which people with diabetes engage in self-care, and access to professional dental services. Three animal models of oral disease in diabetes have suggested a relationship of dental caries and diabetes mellitus. At about 10 months of age, male WBN/KobSIc rats develop spontaneous diabetes mellitus secondary to pancreatitis. Caries, alveolar bone loss, and periapical bone loss were assessed radiographically and histologically in male diabetic WBN/KobSIc animals and unaffected female controls [1]. The caries scores were significantly higher for the male diabetic rats. The difference was most pronounced in the maxilla. Alveolar bone loss and periapical periodontitis (indicative of pulpal necrosis that resulted from extensive caries) were also evident in the male animals. The WBN/KobSIc rats develop dental caries even in the absence of a cariogenic diet or inoculation with cariogenic bacteria, so this is a model that clearly favors the development of tooth demineralization. While the authors conclude that the hyperglycemia is the cause of the increased dental disease, they did not investigate or suggest specific reasons to account for this increased susceptibility. In another animal study, a murine model was used to assess the relationship of hyperglycemia and dental caries [2]. The Akita -/- murine model is similar to type 1 diabetes mellitus. The animals demonstrate reduced insulin production, retinopathy, neuropathy, and early death. Dental caries and salivary flow had not previously been studied in these animals. Controls were nondiabetic mice from the same strain. Tooth morphology and biochemistry, caries development, salivary flow, and histology of the salivary glands were assessed over a nine-week period. The animals with diabetes demonstrated altered tooth morphology and hypomineralization. Subsequently pulpal abscesses developed, leading to necrosis of the pulp and apical bone loss (periapical periodontitis). When challenged with the drug pilocarpine, the non-diabetic animals produced copious amounts of saliva while the diabetic animals produced virtually none. Gross morphology of the salivary glands did not reveal differences for the diabetic animals, but histologic analysis suggested that diabetes led to reduced granule secretion, implying a neuropathy. Another murine model was used to study the relationship of diabetes mellitus to dental caries in type 2 diabetes mellitus [3]. The db/db mouse model is characterized by obesity and early evidence of elevated insulin levels in blood. This model has been used to evaluate both nephropathy and neuropathy as complications of diabetes. The db/+ mice served as controls, because these animals do not manifest diabetes mellitus. Both extent and severity of dental caries were pronounced in the db/db mice. At one year, dental caries was present in 85% of animals with diabetes, but only 22% of controls. In this model dental caries was associated with gingival inflammation, as well as pulpal involvement and apical periodontitis. The literature examining the occurrence of coronal caries in patients with diabetes mellitus contains conflicting reports of increased caries compared to age-matched controls, as well as no differences or even decreased caries in affected individuals. A number of studies have found an increased caries incidence in patients with type 1 diabetes mellitus [4–9]. These studies generally focus on children and adolescents, and two of the studies examined salivary flow as part of the analysis. Reduced flow, and lower salivary pH, has been reported in patients with diabetes [4]. Another study did not observe differences in salivary flow or buffering capacity [9]. Higher caries rates have also been reported in patients with type 2 diabetes mellitus [10–16]. Type 2 diabetes is primarily a disease of adults, and that was reflected in the age of the patients. Differences were observed primarily for root caries, and less so for coronal caries. Two of the studies examined salivary flow and chemistry [10, 11], and both suggested reduced production of saliva, lower concentrations of calcium and phosphate ions, and lower salivary pH. In contrast, an approximately equal number of studies of both children and adults with type 1 and adults with type 2 diabetes mellitus failed to find differences in caries when compared with controls [17–25]. What is clear is that the studies differ in terms of populations and methodology. Furthermore, there are a number of important confounders that ideally should be considered when examining the prevalence and incidence of dental caries in patients with diabetes mellitus [26]. These can be considered as primary and secondary. Primary confounders include the patient’s metabolic status, the use of xerogenic medications, and presence of periodontal disease (primarily in adults, which is associated with gingival recession and greater exposure of susceptible root surfaces). A number of studies have examined the relationship of dental caries to metabolic control of diabetes mellitus. Children or adults with diabetes and poor metabolic control were compared to children or adults who are diabetic and demonstrate good control. While a limited number of studies report no effect of metabolic control on the caries rate [23, 27, 28], a greater number of studies report an increased caries rate in poorly controlled vs. well-controlled patients [29–34]. In summary, unlike the situation for periodontitis, caries is not a recognized complication of diabetes mellitus. Some children with type 1 and type 2 diabetes can be expected to demonstrate a high caries rate, especially if the diet is high in cariogenic food. For adults, it appears that the prevalence of root caries is higher in patients with diabetes versus age-related controls (Figure 8.1). This may relate to exposure of root surfaces secondary to periodontal disease and loss of periodontal tissues. The caries prevalence also may be linked to reduced salivary flow or altered salivary chemistry, which in turn may be secondary to neuropathy affecting the salivary glands. The situation is further complicated by the fact that patients with diabetes mellitus do not access oral health care services as frequently as individuals without diabetes mellitus, and studies suggest that patients with diabetes mellitus are not knowledgeable about the oral complications of the disease (see below). Figure 8.1 Root caries in a patient with diabetes mellitus. Xerostomia is also present. Courtesy of Dr. Angus Walls. The caries risk for patients with diabetes should be assessed and managed on an individual basis. Personalized recommendations for oral hygiene and self-care, diet, and the appropriate schedule of professional visits should be provided. Saliva is an exocrine secretion, and has a number of important functions in the oral cavity. Mixing with the bolus of food, saliva contains amylase, which begins the digestion of starch in food. Saliva also has an important role in maintenance of homeostasis in the oral cavity. This fluid contains antimicrobial substances, and has a buffering capacity that neutralizes acid produced by cariogenic bacteria. The lubricating function is also important; individuals with hyposalivation often complain of discomfort, an inability to eat, and a lack of enjoyment from eating. Saliva is produced by three sets of major salivary glands: the parotids (the largest, located laterally and below the ear and lateral to the ramus of the mandible), the submandibular (located below the mandible and tongue), and the sublingual (located below and to the sides of the tongue and anterior to the submandibular glands). The parotid glands empty into the oral cavity through Stenson’s ducts located in the cheek, at about the line where the teeth occlude. The submandibular glands empty into the oral cavity at Warton’s ducts, located on the ridge of tissue at the anterior of the floor of the mouth. Saliva from the sublingual glands enters the oral cavity through a diffuse ductal system. There are also hundreds of minor salivary glands distributed throughout the mouth, in the submucosa, and found primarily on the soft palate, interior of the lips and vestibules, and buccal surfaces. In a normal adult, approximately 700 ml of saliva are produced in a 24-hour period. Salivary hypofunction and the complaint of xerostomia have been repeatedly demonstrated in patients with diabetes mellitus. This finding has been reported in patients of all ages. Adolescents with type 1 diabetes were shown to have reduced salivary flow, and mouth dryness was a more common complaint on a quality of life questionnaire. Quality of life for these adolescents with diabetes was also related to their dental caries experience [35]. Similar findings were reported in a cohort of diabetic patients with a mean age of 33 years [36]. Both subjective and objective assessments indicated that patients with type 1 diabetes mellitus were affected by mouth dryness more frequently than controls (15.8% vs. 10.3%, p = 0.047) and were also shown to have reduced salivary flow rate at rest (0.22 ± 0.014 mL/minute vs. 0.28 ± 0.016 mL/minute, p = 0.005) and when saliva was stimulated (0.89 ± 0.047 mL/minute vs. 1.02 ± 0.054 mL/minute, p = 0.071). Differences were even more pronounced when stratified by very low resting and stimulated flow rates (< 0.10 mL/minute). Both the use of medications that have reduced salivary flow as a side effect, and a higher fasting plasma glucose level, were associated with reduced flow in patients with diabetes. Of interest, they found that of the medical complications of diabetes, only peripheral neuropathy was associated with reduced salivary flow. Similar findings were reported by other investigators [37]. Xerostomia was present in nearly twice as many individuals with diabetes (62%) as compared to people without diabetes (36%; p = 0.001). When salivary flow was measured, reduced flow was seen in 46% of patients vs. 28% of controls. Another study is noteworthy because potentially confounding variables were considered [38]. Patients using medications that were potentially xerogenic were excluded, and lacrimal fluid and other measures of exocrine function, indicating dryness on other mucosal surfaces, were assessed. Here 43% of the patients with diabetes complained of xerostomia, and more than 80% of those individuals were women. Patients with a complaint of xerostomia also reported ocular and vaginal dryness. Xerostomia was associated with a reduced resting salivary flow rate, but no differences were seen between patients and controls for the stimulated flow rate. The reduced flow was inversely related to the level of HbA1c, but was not related to function of the autonomic nervous system. Continuing along the life course, salivary flow has been examined in older patients [39]. All individuals were at least 60 years of age, independent, and lived in the community. Of the 315 individuals who were examined, 52 reported that they had diabetes mellitus. The occurrence of dry mouth and reduced salivary flow was high. For the later, the percentage approached 50% in both unstimulated and stimulated conditions. It is important to note that while the majority of reports observed that xerostomia is a complication of diabetes mellitus, this finding is not universal [40]. A reduction in salivary flow adversely affects quality of life. Adolescents with complaints of a dry mouth and observed hyposalivation had lower scores on the Oral Health Impact Profile assessment scale compared to adolescents with type 1 diabetes but without xerostomia/hyposalivation [41]. While reduced salivary flow and complaints of oral dryness are seen in patients with diabetes mellitus, the relationship of this finding to other important measures of diabetic status is not clear. Compared to people without diabetes, lower salivary flow rates have been reported for individuals with both well-controlled and poorly controlled diabetes mellitus. The reduction in saliva flow was not related to metabolic control as assessed by glycosylated hemoglobin or fasting plasma glucose [42]. In contrast, other studies [43, 44] found a relationship between salivary flow and glycemic control. Patients with poorly controlled diabetes mellitus demonstrated lower stimulated parotid salivary flow than patients with well-controlled diabetes. Interestingly, there was no correlation of reduced salivary flow with patient complaints of mouth dryness. Therefore, both qualitative and quantitative assessment of salivary flow may be warranted when there is concern about xerostomia in patients with diabetes. In an interesting study that sheds light on the relationship of diabetes mellitus and xerostomia, children and adolescents with newly diagnosed type 1 diabetes were evaluated [45]. Examining salivary flow upon initial diagnosis and the two weeks after initiation of treatment with insulin, increased stimulated salivary flow was seen with establishment of better glycemic control (5.4 ± 3.3 ml/5 minutes vs. 7.3 ± 2.6 ml/5 minutes; p < 0.01). These data argue that xerostomia associated with diabetes is directly linked to serum levels of glucose and not to peripheral neuropathy, a late-occurring complication of diabetes mellitus. The presence of diabetes changes the chemistry of saliva. The most common alteration is an increase in the concentration of glucose. In some studies that increase has been linked to the concentration of glucose in blood [46], and in other studies no association was observed [42, 45]. The elevated levels of glucose in saliva have been associated with an increase in some constituents of the oral microflora (Candida spp), but not others (Streptococcus mutans, lactobacilli; [45, 47]). Other alterations in salivary chemistry have been examined in patients with diabetes. Elevated levels of total protein and urea in saliva were observed in patients with the disease [46]. Furthermore, increased concentrations of antimicrobial constituents in saliva (i.e., lactoferrin, myeloperoxidase, and secretory IgA), have been reported when diabetes is present [45]. In both cases, the increase in concentration of constituents may actually reflect the decreased production of fluid. Inflammatory mediators in saliva from patients with diabetes have also been examined. This analysis is potentially important considering the pro-inflammatory nature of diabetes mellitus, and the increased prevalence of periodontitis in patients with diabetes (Chapter 6). Periodontitis is associated with production of increased gingival crevicular fluid (GCF), which has the characteristics of an inflammatory exudate when periodontitis is present. GCF enters the oral cavity via the gingival crevice, and constituents of GCF are routinely detected in saliva. Matrix metalloproteinase (MMP) levels has been evaluated in saliva from patients with diabetes and controls. Diabetes patients demonstrated both poor metabolic control (mean HbA1c of 8.7%) and increased periodontal disease [48]. The concentration of MMP-8 was elevated in saliva from patients with diabetes who also demonstrated periodontal disease. MMP-8 is derived from polymorphonuclear leukocytes (PMN), which are important cells in the acute inflammatory response in the gingiva. Furthermore, a study examining protease levels in serum, urine, and saliva from patients with diabetes observed higher levels of MMP-2 (which degrades type IV collagen) and MMP-9 (which degrades the extracellular matrix) in serum from patients with diabetes [49]. They also reported higher levels of MMP-9 in saliva from patients with diabetes mellitus who also demonstrated microvascular complications (nephropathy, retinopathy). Other reports have noted that as compared to controls, patients with diabetes mellitus have lower salivary levels of the antimicrobial glycoside hydrolase lysozyme [50], as well as lower levels of epidermal growth factor [51], suggesting a reduced mucosal healing capacity in the oral cavity. One of the most often mentioned oral complications of diabetes mellitus is Candida infection. Candida is a genus of yeast, and Candida albicans is the most common species that exists as a commensal organism in the oral cavity and gastrointestinal track. In the unperturbed individual, Candida does not cause disease. However, Candida albicans infection is observed with immunosuppression, or when homeostasis has been altered. C. albicans infections are seen in patients with HIV/AIDS, when malignancy is present and patients receive cancer chemotherapy, and in patients with poorly controlled diabetes mellitus. Candida infection can also occur in patients who receive an extended course of treatment with antibiotics or corticosteroids. Not unexpectedly, oral Candida infection, and the clinical manifestations of this infection, are influenced by many variables. It has been proposed that in diabetes mellitus, elevated levels of glucose in blood and the tissue promote growth of Candida [52]. Elevated levels of glucose in blood have been examined as a risk factor for oral candidiasis. Evaluating patients with type 2 diabetes, a positive relationship was seen between the concentration of glucose in saliva and the presence of Candida in the oral cavity [53]. Confirming other work, the concentration of glucose in saliva from patients with diabetes was higher than that of controls. The number of Candida colony forming units correlated with the glucose concentration in unstimulated and stimulated saliva, as well as in the serum glucose concentration in patients with diabetes. Furthermore, the reduced phagocytosis and killing capacity of PMN observed in people with diabetes has also been proposed to explain Candida overgrowth [54]. A study of PMN function in patients with diabetes mellitus revealed reduced cell function (phagocytosis, oxygen radical production, and intracellular killing of Candida) when diabetes and Candida infection were both present. Of interest, following treatment of the fungal infection, the reduced PMN function improved, but not to the level of the normal/control cells. The authors suggest that with any case of unexplained oral infection (fungal or odontogenic), clinicians should consider assessing the blood glucose level to rule-out diabetes mellitus [55]. The saliva of patients with diabetes has an elevated concentration of glucose. The constant bathing of the mucosal surfaces in saliva may account for the higher prevalence of Candida in the oral cavity of individuals with diabetes. This bathing does not occur in the esophagus, and consequently a lower prevalence of esophageal vs. oral candidiasis is seen in patients with diabetes [56]. There is an increased carriage rate of oral Candida in patients with diabetes who were treated with insulin [57]. Using a rinse technique, Candida was detected in 77% of patients. The species most frequently identified was C. albicans. Clinically, erythematous candidiasis was the most common form of candidiasis, but 40% of those with microbiologically detected candidiasis did not display a clinical lesion. The significant risk factor for an increased carriage rate was cigarette smoking, and the presence of a denture approached significance. Confirmatory findings in patients with insulin-dependent diabetes mellitus have been reported [58]. Patients with diabetes were five times more likely to demonstrate clinical signs of candidiasis than were controls (15.1% vs. 3%). These manifestations included angular cheilitis, median rhomboid glossitis, and denture stomatitis. Taking a cytological smear, pseudohyphae (indicative of yeast infection) were found nearly four times as often in patients with diabetes vs. controls (23% vs. 5.7%), and when detected, higher pseudohyphae counts were seen for patients with diabetes. Risk factors for pseudohyphae in the cytological smear included cigarette smoking (OR = 2.4), the presence of a denture (OR = 2.3), and poor glycemic control as indicated by an elevated level of HbA1c (OR = 1.9). Other variables were not significant risk factors, including the use of potentially xerogenic medications. Nevertheless, while certain risk factors for Candida infection and Candida overgrowth in the oral cavity have been identified, not all studies have been able to confirm these findings [59, 60]. The species of Candida that is most frequently isolated from the oral cavity in healthy individuals and patients with diabetes mellitus is Candida albicans. However, other species of Candida can be detected in the oral cavity. The species of Candida in the oral cavity of patients with type 1 diabetes, type 2 diabetes, and controls has been examined [61]. Using salivary samples, Candida albicans was the most frequently detected species in all groups, but additional species detected in patients with both type 1 and type 2 diabetes mellitus were C. stellatoidea, C. lipolytica, C. parapsilosis, C. krusei, C. tropicalis, and C. glabrata. C. kefyr was detected in one control patient. The susceptibility of the isolates to different antifungal medications was also examined. All isolates were susceptible to flucytosine and amphotericin B, but a limited number of isolates from type 2 diabetes mellitus were resistant to ketoconazole. The different species of oral Candida present in patients with diabetes has been examined with different levels of metabolic control [62]. As in other studies, C. albicans was the most prevalent species in both patients with poorly controlled diabetes (53% positive) and well controlled diabetes (33%). Other species detected in the poorly controlled patients were C. glabrata (20%), C. tropicalis (6%), and C. parapsilosis (6%). The non-albicans species detected in the well-controlled patients was C. glabrata (13%). C. tropicalis and C. parapsilosis were not present in this group. There has been interest in the presence of C. dubliniensis in the oral cavity of patients with diabetes. C. dubliniensis is a species of Candida identified in the oral cavity of immunosuppressed patients with human immunodeficiency virus [63]. It is believed that the natural habitat for this organism is the oral cavity. There is evidence for the presence of C. dubliniensis in patients with diabetes mellitus [57, 64], but another study failed to identify this species in the oral cavity of patients with diabetes [62]. One important risk factor for clinical Candida infection in patients with diabetes mellitus is the presence of a removable denture. This is observed on the oral mucosal surfaces of patients with complete dentures and removable partial dentures. The Candida infection tends to occur on the mucosal surface below the acrylic portion of the denture, and both tissue inflammation and the carriage rate of Candida infection in those patients has been examined [65]. The prevalence of denture stomatitis was greater in patients with type 2 diabetes vs. healthy controls. While the percentage of individuals with diabetes who were colonized by Candida was higher than that of the normal controls, this difference was not significant. However, the concentration of Candida infection was greater in the individuals with diabetes. Furthermore, C. albicans showed greater adherence to palatal epithelial cells from patients with diabetes compared to controls. The increased adherence of Candida albicans to buccal epithelial cells from patients with diabetes had previously been demonstrated [66]. Colonization of the underside of maxillary dentures by C. albicans in edentulous patients is increased when diabetes is present. This increased density correlated with higher serum glucose levels and the duration of denture use [67]. A study of denture stomatitis in patients with type 2 diabetes mellitus revealed an increased prevalence of stomatitis in patients with diabetes vs. healthy edentulous individuals (57% vs. 30%; p = 0.002) [68]. Accompanying the denture stomatitis was a more than two-fold increase in clinical complaints, primarily of a burning sensation. Oral dryness, glossitis, and angular cheilitis were also much more commonly observed in patients with diabetes. The subjective and objective symptoms of denture stomatitis were associated with higher serum levels of HbA1c (Figure 8.2). This study also suggested that a number of mucosal symptoms occurred concurrently in patients with diabetes and diffuse inflammation. These include burning mouth syndrome and oral dryness, and to a lesser degree traumatic ulcers, angular cheilitis, and glossitis (Figure 8.3). Figure 8.2 Mean HbA1c and type of mucosal inflammation. Mean HbA1c values (and 25th and 75th percentiles) for edentulous patients with type 2 diabetes mellitus and different types of mucosal inflammation (DS I = localized simple inflammation, DS II = diffuse inflammation, DS III = localized hyperemic inflammation) [68]. Figure 8.3 The number of complete denture patients with different oral symptoms. When diabetes mellitus was present, symptoms were markedly increased (BMS = burning mouth syndrome, DR = oral dryness, TRU = traumatic ulcer, ACH = angular cheilitis, GL = glossitis) [68]. The clinical appearance of Candida infection in the mouth can vary markedly. The lesions include acute pseudomembranous candidiasis, acute atrophic candidiasis, median rhomboid glossitis, chronic atrophic candidiasis (denture stomatitis), chronic hypertrophic candidiasis, and angular cheilitis. Acute pseudomembranous candidiasis is characterized by erythematous (red) areas with milky white to light yellow “curds” (Figures 8.4 and 8.5). The “curds” are aggregations of fungal organisms. Acute atrophic candidiasis is characterized by an erythematous mucosa but without the aggregations of fungal organisms. Acute pseudomembranous candidiasis may transform into acute atrophic candidiasis as the fungal infection resolves with treatment (Figure 8.6). In both acute pseudomembranous candidiasis and acute atrophic candidiasis, the dorsal surface of the tongue may appear red and atrophic (referred to as “bald” and also known as median rhomboid glossitis). This is due to the temporary loss of the filiform papillae (Figure 8.7). Chronic atrophic candidiasis appears as a red mucosal surface beneath a removable partial or complete denture. The erythema follows the outline of the prosthesis (Figures 8.8 and 8.9), and has also been referred to as “denture stomatitis.” Chronic hypertrophic candidiasis is a lesion that reflects a chronic Candida infection. The persistent infection causes a mucosal response characterized by hyperplasia and hyperkeratosis. Here the white patch is adherent and cannot be removed (leukoplakia; Figures 8.10 and 8.11). This is often a difficult clinical diagnosis. Angular cheilitis is seen at the corner of the lips. Because these lesions demonstrate Candida when a smear is examined or when a culture is taken, they are considered a form of Candida infection (Figure 8.12). It is important to emphasize that more than one type of clinical lesion can be seen in a patient. The most common complaint of patients with an intraoral Candida infection is a burning sensation, or report of a very sensitive mouth. Figure 8.4 Acute pseudomembranous candidiasis of the tongue. Courtesy of Dr. David Zegarelli. Figure 8.5 Acute pseudomembranous candidiasis of the buccal mucosa. Courtesy of Dr. David Zegarelli. Figure 8.6 Acute atrophic candidiasis of the tongue. Courtesy of Dr. David Zegarelli. Figure 8.7 Median rhomboid glossitis. Courtesy of Dr. David Zegarelli. Figure 8.8 Chronic atrophic candidiasis (denture stomatitis) of the hard palate. Courtesy of Dr. David Zegarelli. Figure 8.9 Chronic atrophic candidiasis (denture stomatitis) of the hard palate. Courtesy of Dr. David Zegarelli. Figure 8.10 Chronic hypertrophic candidiasis of the buccal mucosa. Courtesy of Dr. David Zegarelli. Figure 8.11 Histology of chronic hypertrophic candidiasis. Epithelium is thickened, and pseudohyphae are seen in the cornified layer. Courtesy of David Zegarelli. Figure 8.12 Angular cheilitis. Courtesy of David Zegarelli. Burning mouth syndrome (BMS) is a complaint reported by patients with diabetes mellitus, but is not unique to patients with diabetes. Furthermore, as a symptom, there appear to be a number of underlying causes for BMS. There is often no clear cause for BMS. Patients complain of burning or irritation of the mouth, often primarily involving the tongue. Taste disturbances can accompany a complaint of burning mouth, as can a subjective or objective complaint of mouth dryness. A review of BMS concluded that the disorder is defined as a chronic condition characterized by a sensation of burning of the oral mucosa and/or tongue that occurs spontaneously, can be intense, and is not accompanied by any obvious clinical lesion that would account for the symptoms. The prevalence ranges from 0.7%–4.6% with post-menopausal women most often affected. Both idiopathic forms (referred to as primary BMS) and secondary BMS are recognized. The secondary forms are associated with an identifiable cause. Delays often occur in both the diagnosis and referral for appropriate treatment [69]. BMS is found in association with a number of disorders, including menopause, diabetes mellitus, Candida infection, mental illness including depression, Sjörgrens’s syndrome, and contact sensitivity to a product or component of a product that is used in the mouth, mainly toothpastes [70]. BMS in patients with diabetes has been examined as a manifestation of neuropathy. A study of tactile sensory function in patients with BMS suggested that affected individuals could be grouped into different categories based on electrophysiologic findings, with particular attention to the blink reflex and the presence of trigeminal neuropathy. This study excluded patients with mucosal lesions or signs of Candida
Non-periodontal oral complications of diabetes mellitus
Introduction
Dental caries
Xerostomia
Candida infection
Burning mouth syndrome
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree