The liver is one of the most common sites of metastatic involvement for not only gastrointestinal malignancies, but also for a broad spectrum of cancers. While for gastrointestinal malignancies the pattern of blood flow via the splanchnic system seems an obvious explanation, many other malignancies (ocular melanoma and medullary thyroid carcinoma as examples) must offer other explanations likely revolving around the unique environment present with the liver. Local interventions including partial hepatectomy have been advocated for cancers whose dominant site of metastasis involves the liver, including colorectal cancer and gastrointestinal neuroendocrine cancers. Early experiences in partial hepatectomy and other local interventions for metastatic colorectal cancer defied commonly held (and largely accurate) perceptions that as a systemic matter cure would be unlikely. Although randomized trials comparing resection to observation or systemic therapy alone do not exist, large experiences have contributed to a general acceptance of the role for local hepatic treatments in metastatic colorectal cancer when confined to the liver or even in the presence of controlled, minimal volume at extrahepatic sites. This argument is supported by the positive 5-year survival rates after resection of colorectal liver metastases that is reaching 40% to 71%.1–5 Local treatments for hepatic metastases are also generally accepted in patients with gastrointestinal neuroendocrine malignancies, given the fact that hepatic dominant pattern is present in many patients and that uncontrolled hepatic tumor burdens are often the cause of ultimate demise in these otherwise “indolent” or slowly progressing malignancies.
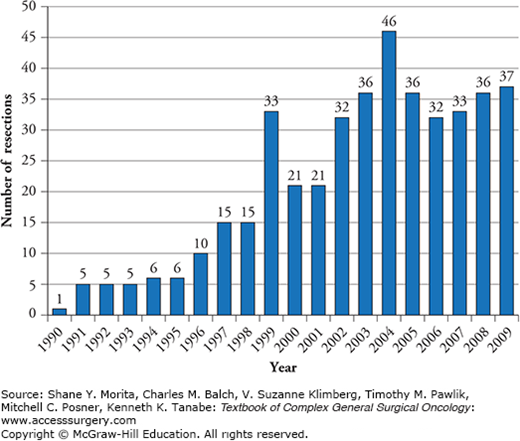
Historically, hepatectomy in patients with noncolorectal nonneuroendocrine liver metastases (NCNNLMs) was considered to be less beneficial compared to patients with liver metastases originating from colorectal cancer or neuroendocrine tumors. Early experiences in hepatic resection for liver metastases arising from pancreas (adenocarcinoma), breast, lung, stomach, kidney, reproductive organs, and cutaneous sites led many authors to suggest a limited role, if any for this modality.6 However, this notion has been increasingly challenged during the last two to three decades. A number of factors have contributed to this reconsideration, including improvements in imaging, surgical techniques, adjuvant systemic treatments, and the overall morbidity profile of hepatic surgery. There was a threefold increase in partial liver resections for NCNNLMs in four major hepatobiliary centers in the United States comparing years 1990–1999 to 2000–2009 (Fig. 131-1) and even a fourfold increase from 1983 to 1993 compared to 1994 to 2004 in France.7,8 Notwithstanding, there are no guidelines or specific criteria as to which patients with NCNNLMs should undergo partial liver resection.
Figure 131-1
Number of partial liver resections per year for noncolorectal nonneuroendocrine liver metastases at four specialized hepatobiliary centers in the United States. (Reproduced with permission from Groeschl RT, Nachmany I, Steel JL, et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. May 2012;214(5):769–777.)
This chapter aims to summarize the current knowledge about treatment strategies of NCNNLMs from a surgical perspective.
Liver metastases are 18 to 40 times more common than primary liver cancer. While liver dominant-disease is most often associated with colorectal cancer and gastrointestinal neuroendocrine malignancies, NCNNLMs are associated with almost all potential primaries including origin in the gastrointestinal tract like pancreatic, oesophageal, and gastric cancer, breast cancer, lung cancer, melanoma, sarcomas, and tumors from the genitourinary system like renal cell carcinoma, endometrial, testicular, and ovarian cancer. Very rarely, the primary tumor cannot be discovered even after extensive investigations and is then called cancer of unknown primary. The historical “adenocarcinoma of unknown primary” is much less a frequent conclusion with improved pathologic techniques that accurately diagnose intrahepatic cholangiocarcinoma.
A number of investigators have explored the pattern of intrahepatic distribution of metastases. Among patients with NCNNLMs who underwent a resection of the metastatic hepatic lesion, there does not appear to be a predilection of the tumor location. In a report by Yedibela et al,9 liver metastases were distributed in the right lobe in 38%, in the left lobe in 35%, and bilobar in 27% of the patients. Others have supported this lack of preferred localization.7,8,10 The timing of the identification of metastasis in patients undergoing resection of NCNNLMs has also been described by a number of authors with apparent selection bias toward patients with metachronous presentations.7,9,11,12 In several patients (14.4%), liver metastases were even diagnosed before resection of the primary tumor.7 In this same study by Groeschl et al, the mean time between resection of the primary tumor and diagnosis of liver metastases was 43 months (range: 0 to 312 months), while it was 38 months (range: 0 to 448 months) in a study by Adam et al.8 Among those patients, 12.4% had their liver metastases detected during surgery for resection of the primary tumor. If liver metastases were synchronous, 39.3% were resected during the same surgery as for the primary tumor.12 In a study performed at the Mayo Clinic, only 15% of the patients underwent concomitant resection of the primary tumor and the liver metastases.10 This difference might reflect different treatment strategies but might also be due to different referral patterns as patients with complex diseases are often referred to a tertiary center after preliminary workup and already initiated treatment for the primary.
When considering the proper place of hepatic resection in the treatment plan, an important consideration is the patient’s fitness for surgery. While this seems obvious in many patients, this decision is often made utilizing improper perceptions about the morbidity profile of resection and the magnitude of medical comorbidities and advanced age on perioperative outcomes. In general NCNNLM patients presenting with very poor performance status arising from significant comorbidities or from the malignant process should not be considered as surgical candidates. In the current era, well-managed comorbidities including coronary artery disease, chronic renal insufficiency, and diabetes (as examples) do not represent absolute contraindications, nor should “advanced” ages in otherwise robust patients.
Some investigators found that a longer interval between primary tumor resection and the development of metastases is a surrogate for favorable tumor biology.8,13–17 This idea, however, did not have an impact on long-term survival in the study by Groeschl et al.7 Patients age 60 or older, those with primary tumors other than breast cancer, melanoma, or squamous cell cancer, were associated with worse outcome compared to their counterparts.8 In contrast, a higher administration of preoperative and postoperative chemotherapy was significantly associated with improved survival.7 While those factors can be evaluated preoperatively, other characteristics associated with inferior long-term survival are harder to assess before surgical completion including a larger tumor size (e.g., >5 cm), lymphovascular invasion, presence of extrahepatic nodal disease, and R2 resection.7,8,14–16,18,19 Based on the characteristics of their patient’s cohort, Adam et al8 developed a prognostic model stratifying patients into low-risk, mid-risk, and high-risk category, with 5-year survival times of 46%, 33%, and <10%, respectively. They included the following six variables into the model: extrahepatic metastases present prior to or at the time of hepatectomy (0/1 point), major hepatectomy (0/1 point), R2 resection (0/1 point), patient age (0 to 2 points), length of disease-free interval between treatment of the primary tumor and diagnosis of metastases (0 to 2 points), and primary tumor characteristics (site and histology; 0 to 4 points). Lendoire et al did apply this risk score to his patient cohort and found that 5-year survival in the high-risk group was 0, 8% in the mid-risk group, and 31% in the low-risk group.14 In addition, blood loss during surgery was accused to be a risk factor for worse survival.7 The data reported by Yedibela et al9 does support this finding showing that patients who received less than 5 packed red blood cells had a longer median survival of 26 months than patients who needed ≥ 5 packed red blood cell products who lived only 11 months in median. Whether or not this is an independent consequence of the decreased amount of blood loss/need for blood products or whether this is just a co-finding of overall improvements over time is unclear. Groeschl et al7 highlight that during the years 2000 to 2009, the median blood loss was 250 mL (interquartile range 100 to 500 mL), while it was 500 mL (interquartile range: 200 to 800 mL) during the years 1990 to 1999, p = 0.01.
Despite the already documented patient and tumor characteristics associated with survival, additional research has to be performed so that patients benefit from an aggressive surgical approach. Until now, the selection of surgical candidates should follow a broad and multidisciplinary discussion by all-involved specialty teams trying to outweigh benefits and risks of this patient population.
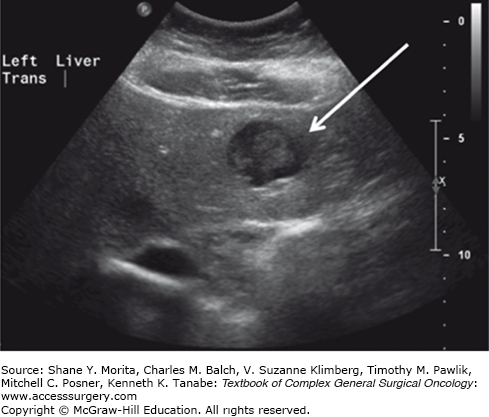
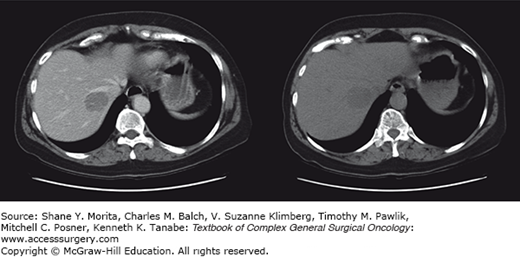
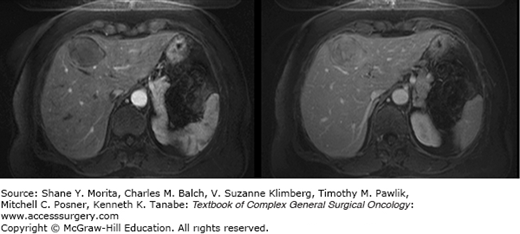
State-of-the-art imaging is critical to arriving at the optimal treatment plan in patients with suspected hepatic metastases (see Chapter 123). Although contrast-enhanced CT and MRI imaging are the optimal modalities for defining the burden of disease within the liver in most malignancies, they may not be best for the delineation of extrahepatic tumor burdens in many types of histologies. As such, the proper imaging strategy to define tumor burdens in constructing treatment plans is dependent upon the specific histology (Figs. 131-2 to 131-4). For many disease (melanoma, lung cancer, gastrointestinal stromal tumor (GIST), and others) positron emission tomography (PET) imaging may complement MRI- and CT-based cross-sectional imaging of the liver to best define the extent of disease. Over the past two decades, there has been a tremendous increase in the accuracy of liver tumor detection.20-22 Most of the studies evaluating the accuracy of those diagnostic tools were performed evaluating colorectal liver metastases due to its high prevalence. While contrast-enhanced sonography did improve the performance and detection rate of liver metastases, multidetector-computed tomography does still outperform contrast-enhanced ultrasonography in some, but not in all studies.23–25 Due to its wide availability, its fast realization, the detailed representation of the intrahepatic anatomy necessary to assess potential resectability, and its reproducibility and investigator independency, multidetector-computed tomography is still the favorable technique to assess most of the patients (Fig. 131-2). To best detect and differentiate even small lesions, four distinct phases (native, arterial, portal-venous, and late venous) should be performed. CT scan even allows the fast assessment of additional metastatic lesions in the abdominal cavity or the lung. The development of contrast-enhanced ultrasonography has significantly improved the sensitivity in detection of liver metastases compared to conventional ultrasonography (Fig. 131-3).26 The ability to characterize focal liver lesions given the chance of real-time evaluation of the liver perfusion has significantly improved the sensitivity to detect liver metastases. However, contrast-enhanced ultrasonography has its inherent limitations: the quality of the examination heavily depends on the skill of the operator, not all parts of the liver are well visualized, only one lesion at a time can be examined in the arterial and early portal-venous phase, and difficulties may arise in distinguishing hypervascular metastases from hemangiomas. In cases where difficulties arise in distinguishing very small hypervascular lesions from benign processes such as hemangiomas, cysts, and nodular hyperplasia, MRI can be particularly helpful (Fig. 131-4).
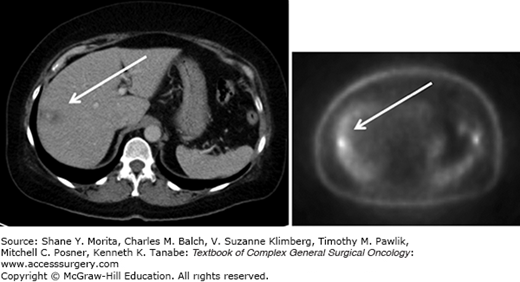
Positron emission tomography is a functional technique highlighting lesions with increased glucose metabolism combined with a CT to get cross-sectional images (Fig. 131-5). While the liver itself is an organ with a high baseline glucose metabolism and its breath-dependent movement inducing artifacts, the detection of small lesion in the liver is difficult. However, already around the year 2000, studies showed that clinical management decisions were changed in about 20% of the patients with colorectal liver metastases based on PET-scan results.27,28 Newer PET-CTs even have the option of acquiring respiratory gated PET images which reduce respiratory artifacts helping to more accurately identify liver lesions.29 PET-CT scans are often used to detect distant metastases aside of the primary tumor lesion and the liver metastases, and advances in its technique and accuracy will even increase its use. Patients with uncontrolled extrahepatic metastatic disease might not be candidates that benefit from partial liver resection.
Besides cross-sectional imaging, staging laparoscopy can be an important adjunct in the decision process of whether or not a patient with NCNNLMs should undergo open exploration and potentially resection. D’Angelica et al30 evaluated a series of patients as candidates for partial liver resection from 1997 to 2000. They assessed 30 patients who were considered resectable after CT or MRI, while 70% of the patients had two or more imaging studies performed prior to diagnostic laparoscopy. Twenty-three of the patients underwent simultaneous laparoscopic ultrasound, and 24 patients were considered as being successfully explored. Six of 30 patients were considered unresectable during staging laparoscopy while one of them needed a small laparotomy to achieve safe port placement due to extensive adhesions. The remaining 24 patients underwent open exploration. Three patients were found to be unresectable after laparotomy due to either invasion of the main portal vein or the involvement of all three liver veins. In summary, only taking 30 minutes on average with no morbidity to those having unresectable disease, staging laparoscopy should be advocated for cases where only little doubt of resectability is expressed.
Further improvements in the accuracy of the preoperative detection of liver metastases can be expected as the CT, PET-CT, MRI, and (contrast-enhanced) sonography techniques will further improve. New technological progress permits incorporating such radiological information to allow 3D visualization of the patient-specific internal liver anatomy and tumor location during surgery (Fig. 131-6).31 This computer-based support during surgery improves anatomical orientation through stereotactic navigation technology and even allows displaying tumors that disappeared due to chemotherapy at time of surgery. Image-guided liver surgery is still facing challenges, as accuracy is still often imprecise in parts of the liver prone to deformation of the liver morphology. Next-generation navigation tools might overcome this limitation and will allow patients with NCNNLMs to benefit from this technology.
The past two decades have seen a marked improvement in the morbidity profile of liver resection9,11,13,32–35 (see Chapter 125). This has been documented not just in patients with colorectal and neuroendocrine liver metastases, but also in patients with NCNNLMs. The reasons for those better short-term outcomes are many and include superior preoperative imaging allowing better patient selection, improvements in anesthetic and critical care support, enhanced understanding of the complex liver anatomy, parenchymal preservation strategies, and more comprehensive multidisciplinary algorithms.36,37 Minimization of blood loss with newer resection techniques and the avoidance of major intraoperative events through experience are such that most hepatic procedures should now be considered straightforward and occur with a minimum of excitement. Attention to the viability and adequacy of the future liver remnant has been increasingly recognized as critical to improvements in perioperative outcomes. Adequate vascular portal and arterial inflow and venous outflow must be guaranteed after partial liver resection. In addition, biliary drainage must be ensured either by its natural course or by performing a biliodigestive anastomosis. The volume of the future liver remnant can now be assessed with reasonable accuracy through volumetric analysis of cross-sectional imaging. Depending on the extent of preoperative chemotherapy and the underlying condition of the liver, the future liver remnant must be at least ≥20% of the total estimated liver volume. If extensive chemotherapy has been performed prior to partial liver resection, the future liver remnant should be at least ≥30%. In cases of liver cirrhosis (Child’s class A), recommendations are advocating preservation of ≥40%. In situations where the future liver remnant is judged to be inadequate, its volume can be augmented by preoperative portal vein embolization (of those branches within the planned resection) to induce liver hypertrophy of the remnant.
Many single- and multi-institutional studies have reported experiences with the resection of NCNNLMs. Perioperative mortality rates are reported to be between 0% and 9% (Table 131-1), which is comparable to patients undergoing liver resection for colorectal or neuroendocrine metastases.7,9,11,12,15,19,34,36–41 Overall postoperative complications in patients undergoing liver resections are not explicitly defined and differ from study to study which makes comparison among the reports difficult. As such, the range in operative morbidity is from 0% to 40% (Table 131-1). Reddy et al11 reviewed outcomes with a broad spectrum of postoperative morbidities including death, organ-system failure, sepsis, intra-abdominal abscess, pancreatitis, prolonged ileus, anastomotic leak and stricture, symptomatic pleural effusion, pneumothorax, infection (excluding urinary tract infection), fascial dehiscence, bleeding, seizures, encephalopathy, gastrointestinal bleeding, deep venous thrombosis/pulmonary embolism, cerebral-vascular accidents, and arrhythmias. Patients undergoing liver resection for NCNNLMs in comparison to those undergoing liver resection for colorectal and neuroendocrine metastases experienced no appreciable difference in complication rates.11 Similar experiences have been reported by others highlighting that liver resection in NCNNLM patients can be performed in as safe a manner as in patients with colorectal and neuroendocrine metastases.32,36,37 Reddy et al11 directly compared length of hospital stay between patients with NCNNLMs, and colorectal and neuroendocrine metastases. While no difference was found between patients with NCNNLMs (median: 7 days, range: 3 to 48 days) and colorectal metastases (median: 7, range: 3 to 55), patients with neuroendocrine liver metastases had a significantly longer hospital stay compared to both other groups (median: 10 days, range: 524 days).11
Review of Studies of Patients Undergoing Partial Liver Resection for NCNNLMs; Postoperative Mortality and Morbidity
| Author | Year | Duration | Number of Patients with Neuroendocrine Primaries | Number of Patients with NCNNLMs | Mortality (%) | Morbidity (%) |
|---|---|---|---|---|---|---|
| Harrison et al39 | 1997 | 1980–1995 | 0 | 96 | 0 | – |
| Berney et al107 | 1998 | 1986–1995 | 8 | 26 | 0 | 19 |
| Lindell et al108 | 1998 | 1970–1995 | 12 | 20 | 9 | 25 |
| Elias et al34 | 1998 | 1984–1996 | 27 | 120 | 2 | – |
| Hemming et al109 | 2000 | 1978–1998 | 0 | 37 | 0 | – |
| Benevento et al38 | 2000 | 1988–1998 | 6 | 14 | 0 | 40 |
| Lang et al40 | 1999 | 1983–1993 | 0 | 127 | 6 | 33 |
| Hamy et al110 | 2000 | 1986–1997 | 8 | 27 | 3 | – |
| Buell et al32 | 2000 | 1990–1996 | 6 | 22 | – | 19 |
| Laurent et al111 | 2001 | 1980–1997 | 0 | 39 | 0 | 8 |
| Yamada et al41 | 2001 | 1990–1995 | 0 | 33 | 9 | 21 |
| Takada et al112 | 2001 | 1987–1999 | 0 | 14 | 7 | – |
| Van Ruth et al113 | 2001 | 1991–1999 | 0 | 28 | 0 | 25 |
| Karavias et al114 | 2002 | 1994–2000 | 0 | 18 | 0 | 11 |
| Goering et al115 | 2002 | 1991–2001 | 13 | 29 | 2 | – |
| Cordera et al10 | 2005 | 1988–1998 | 0 | 64 | 2 | 7 |
| Weitz et al13 | 2005 | 1981–2002 | 0 | 141 | 0 | 33 |
| Ercolani et al35 | 2005 | 1990–2003 | 0 | 142 | 0 | 21 |
| Yedibela et al9 | 2005 | 1978–2001 | 15 | 148 | 8/1a | 29 |
| Adam et al8 | 2006 | 1983–2004 | 0 | 1452 | 2 | 22 |
| Teo et al116 | 2006 | 1997–2003 | 0 | 18 | 0 | 0 |
| Earle et al33 | 2006 | 1990–2005 | 18 | 77 | 2 | |
| Lendoire et al14 | 2007 | 1989–2006 | 0 | 106 | 2 | – |
| Reddy et al11 | 2007 | 1995–2005 | 0 | 82 | 4 | 30 |
| O’Rourke et al18 | 2008 | 1986–2006 | 0 | 102 | 1 | 21 |
| Ercolani et al15 | 2009 | 0 | 134 | 3 | 23 | |
| Lehner et al19 | 2009 | 1994–2008 | 0 | 242 | 2 | 21 |
| Groeschl et al7 | 2011 | 1990–2009 | 0 | 420 | 2 | 20 |
| Kalil et al117 | 2013 | 1993–2007 | 0 | 94 | 4 | – |
The single most important outcome to evaluate whether or not a treatment should be implemented in surgical oncology is its impact on overall survival. Unfortunately, given the current literature, it is not possible to distill the independent impact of the resection of NCNNLMs because of the lack of randomized trials. The current literature does therefore rely on retrospective analyses of single- to multi-institutional studies trying to investigate prognostic indicators among patients who underwent partial liver resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree