Why Do We Need New Antiglycaemic Medications and the ‘Diabetes Conundrum’?
Everyone will agree that elevated blood glucose is bad, but why has it been so difficult to show that lowering blood glucose to normal is good? We have not had the same problem showing that lowering elevated levels of blood pressure and low-density lipoprotein (LDL) are beneficial. Hyperglycaemia leads to increases in mortality and cardiovascular disease, but antiglycaemic therapies have not alleviated this excess burden of disease. This is the ‘diabetic conundrum’ and it creates opportunities for new antiglycaemic therapies since the old ones have been lacking.
The purpose of this chapter is to discuss and evaluate new glucose-lowering (antiglycaemic) therapies for type 2 diabetes mellitus (T2DM). Although blood pressure- and LDL-lowering therapies are effective and important diabetic therapies, they will not be included in this discussion.
Definition of Type 2 Diabetes Mellitus: Disease or Risk Factor?
The American Diabetes Association (ADA) definition is a good place to start the discussion: ‘Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction and failure of various organs, especially the eyes, kidneys, nerves, heart and blood vessels.’ Three points in this definition require emphasis, outlined below.1
‘Hyperglycaemia’, and when does T2DM Start?
The difficulty in understanding T2DM is deciding where normoglycaemia leaves off and hyperglycaemia begins. The glycaemic risk for complications probably begins before the glucose level meets the current diagnostic threshold of T2DM. In this sense, hyperglycaemia is more like a risk factor with a continuous rather than dichotomous (i.e. the presence or absence of T2DM) threat to health.
That the therapeutic implications of hyperglycaemia present a graded rather than a dichotomous risk is emphasized by the new AACE treatment algorithm.2 The AACE therapeutic approach emphasizes that increasing levels of hyperglycaemia require graded intensities of treatment. They divide hyperglycaemia into three tiers, recommending monotherapy for the first tier, duotherapy for the second tier and more complex strategies or consideration of insulin for the third tier. This tiered approach is similar to step therapy for different stages of hypertension in the JNC guidelines. In contrast, the ADA does not consider gradations of hyperglycaemia, but instead recommends a stepwise approach to intensification of therapy in T2DM.3 The theoretical downside of ADA’s stepwise versus AACE’s tiered approach is the delay in reaching the desired glycaemic target.
‘Defects in Insulin Secretion and Action’
Halting the progression of these defects and worsening hyperglycaemia is one of the major therapeutic challenges in T2DM. With time, therapy must be intensified in both dose and numbers of medications to offset the steady decline in insulin secretion. One strategy is to use insulin sensitizers; another is to find drugs this increase β-cell function or mass or decrease apoptosis. Newer therapies with these characteristics will be reviewed.
‘Long-Term Damage, Dysfunction and Failure of Various Organs’
Ultimately, it is the complications that we are most concerned about. They can be divided into microvascular, which responds well to antiglycaemic therapy, and macrovascular, for which it has been harder to show benefit.
Microvascular Complications
Small-vessel disease in T2DM is responsible for blindness (retinopathy), renal failure (nephropathy) and lower limb amputations (neuropathy). Glucose-lowering therapy has been proved to reduce these complications (DCCT trial).
Macrovascular Complications
Large-vessel disease includes coronary artery, cerebrovascular and peripheral vascular disease. All of the major antiglycaemic trials have failed to show benefits in large vessel disease or mortality in T2DM (ACCORD,4 ADVANCE,5 VADT,6 UKPDS trials).
Cancer Complications
Less well appreciated is the association of T2DM and cancer. T2DM patients have approximately twofold higher rates of cancer for a variety of organs, including liver, pancreas, endometrium, breast, colon and bladder, and non-Hodgkin’s lymphoma. Potential explanations include the mitogenic effects of insulin (endogenous and exogenous) and underlying metabolic abnormalities such as increased oxidative stress, hyperglycaemia, hyperlipidaemia and obesity. Little is known about the impact of antiglycaemics and cancer, but metformin shows lower cancer rates than insulin and sulfonylurea (SU) therapies.7
Current Adjunctive Therapies for T2DM
Adjunctive Therapy
The main focus of this chapter is antiglycaemic therapies, but the comprehensive treatment approach of T2DM is multi-factorial, as shown in the STENO-2 trial.8 Drugs to control blood pressure and lipid levels are well-established treatments. Pushing these therapies too far, however, has shown their limits, particularly in T2DM with high risk for cardiovascular disease (CVD).9, 10 Even new aspirin guidelines call for tighter criteria for use in T2DM primary prevention.11
Medical Nutrition and Exercise Therapy (MNET)
Medical nutrition and exercise therapy is unquestionably the foundation for T2DM prevention, antiglycaemia and CVD risk reduction. MNET should be prescribed to achieve treatment goals, preferably provided by a registered dietician familiar with diabetes education. The unresolved issue, however, is which target to use as a goal for MNET. Even though a body mass index (BMI) goal of <25 is recommended, this is not supported by the data. Based on BMI, over-weight and obese T2DM patients have the lowest rates of CVD and mortality compared with lower and higher BMI categories.12 The ‘U-shaped’ relationship of BMI and CVD has been termed the ‘obesity paradox’ and probably reflects the fact that BMI does not accurately measure metabolically dangerous fat found in ectopic and intra-abdominal sites. Supine abdominal height, on the other hand, is a simple anthropometric measure and better predicts CVD risk and insulin sensitivity than BMI, waist girth and waist:hip ratio.13
Currently Approved Antiglycaemic Therapies
Most physicians are feeling overwhelmed at the increasing number of antiglycaemics. If one of these drugs did the job, however, we wouldn’t need so many (see Table 102.1). What the problems and needs are for approved and emerging antiglycaemics will be discussed.
Table 102.1 Approved T2DM antiglycaemic therapies.
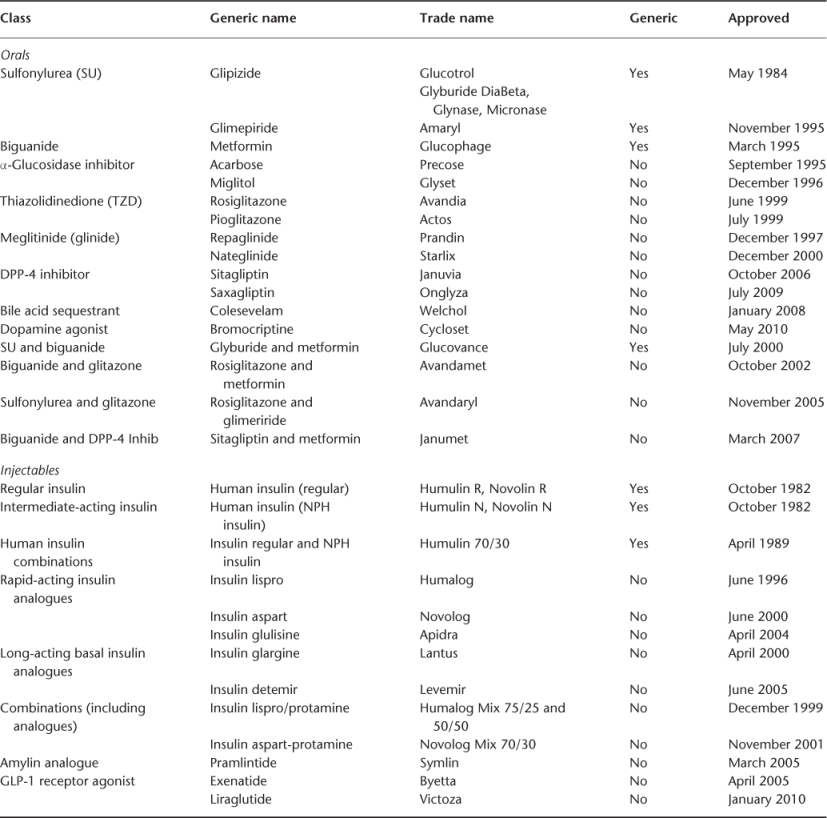
Concerns about the benefits and harms of oral antiglycaemic medication have been with us since the UGDP (University Group Diabetes Program) study in 1970, which reported increased mortality and CVD risk with an SU (tolbutamide). Although in 1998 the UKPDS (United Kingdom Prospective Diabetes Study) was thought to have resolved this controversy, one arm in the study showed increased mortality and CVD risk when metformin was added to an SU (UKPDS34). In 2007, concern was again raised, this time for another drug, rosiglitazone, which was associated with excess mortality and CVD.14
Evaluating the safety and benefits of antiglycaemics is difficult because of the wide variability between classes and the number of drugs in each class. Furthermore, the hard outcomes data beyond glucose lowering are usually not available when making therapeutic decisions.
Even he US Congress is concerned. It directed the Agency for Healthcare Research and Quality (AHRQ), to evaluate the outcomes, comparative clinical effectiveness and appropriateness of prescription drugs, including antiglycaemics. Its report concluded that the evidence from clinical trials about drug efficacy on major clinical endpoints, such as cardiovascular mortality, is inconclusive.15 Therefore, the whole area of T2DM therapeutics is filled with uncertainty. The US Food and Drug Administration (FDA) is making a small step in the right direction since it is requiring all new T2DM drug approvals to examine CVD endpoints and at least show no harm.
Metformin: Life Begins at 50
Metformin is on just about everyone’s list of first-line antiglycaemics. Metformin been around for many years, beginning as Galega officinalis (French lilac) used in mediaeval Europe to treat many medical problems, including diabetes.16 Fifty years ago, metformin was isolated from this plant and introduced for the treatment of T2DM. Since then, it has become the most widely prescribed diabetic therapy and with good reason. Metformin lowers glucose with negligible hypoglycaemia and measurable body weight loss. It decreases microvascular complications and one sub-study of the UKPDS(34) showed a reduction in CVD and mortality as monotherapy in obese T2DM.
Metformin works by preventing hepatic gluconeogenesis through activation of AMP-activated protein kinase (AMPK), which in turn inhibits the expression of other hepatic gluconeogenic genes (e.g. PEPCK, phosphoenolpyruvate carboxykinase). Inhibition of gluconeogenesis lowers glucose production, but also reduces hepatic lactate uptake. Conditions which increase lactate production [alcohol intoxication, low-flow states, congestive heart failure (CHF), respiratory failure] combined with impaired renal clearance will potentially result in lactic acidosis. The most common adverse effects are diarrhoea and decreases in vitamin B12 levels.
Metformin has shown an anti-cancer effect compared with SU and insulin in observational studies.7 The mechanism may be through activation of AMPK, which plays a role in tumour suppression. Beyond the cancer preventive effect, there is evidence that metformin may enhance chemotherapy for existing cancers.17
The bottom line is that this drug deserves to be first-line. It is cheap, effective and has outcomes data. Unfortunately, metformin does not halt T2DM progression and most patients will move on to need another drug; the big question is, which one?
Sulfonylureas (SUs)
‘Special warning on increased risk of cardiovascular mortality’ is how the SU class label reads resulting from the 1970 UGDP study. There is some biological plausibility because SUs block ‘ischaemic preconditioning’, which is a mechanism for protecting the myocardium during periods of ischaemia. SUs bind to the potassium (KATP) channel, leading to an increase in intracellular potassium ion concentrations, thereby opening voltage-gated calcium channels, resulting in an influx of calcium ions. In pancreatic β-cells, this calcium influx promotes insulin secretion, but in the heart it impairs ‘ischaemic preconditioning’.
Most of the physicians who know about the package label warning are reassured by results of the UKPDS33 trial (but not the UKPDS34 trial) and the positive recommendations by the ADA, AACE and other experts. Also, SUs are inexpensive and effective at lowering both glucose and microvascular complications. The good news is that SUs are probably safe, but the bad news is that they do not work to lower CVD and mortality. SUs also do not halt the progression of T2DM, so should we still be using them?
Meglitinides (Glinides): Faster is not Always Better
This class of drugs can be thought of as a rapid-acting SUs. Although they are non-SUs chemically, they act through the same mechanism to stimulate insulin secretion. Because of the rapid onset and short duration of action, glinides are best used to lower postprandial glycaemia (PPG). This means that they have to be dosed before each meal several times per day, which makes them inconvenient. Other negative points include hypoglycaemia, measurable weight gain and lack of positive outcomes data.
The best hope for success for glinides was to show that targeting PPG would improve CVD and prevent progression of T2DM. Previous studies have suggested that PPG was an even greater risk for CVD than fasting plasma glycaemia (FPG) for people with glucose intolerance (DECODE study). Unfortunately, this hope was dashed when pre-meal glinide (nateglinide) therapy failed to show any CVD protection or slowing of the progression of hyperglycaemia.18 Therefore, this appears to be another drug class that is lacking in efficacy.
Thiazolidinediones (TZDs): The Bloom is Off the Rose and the Pie is in the Sky (Rosiglitazone and Pioglitazone)—Downsizing Expectations
At first, this class of drugs promised benefits beyond glucose reduction, as suggested by encouraging surrogate outcomes for CVD. Insulin resistance, interleukin-6 and VEGF-induced angiogenesis all decreased, adiponectin levels rose and visceral fat moved to the periphery. Among the positive attributes, TZDs do not produce hypoglycaemia and they slow the progression of β-cell loss. Compared with SUs and metformin, rosiglitazone has greater durability as monotherapy (ADOPT—A Diabetes Outcome Progression Trial).
The bloom fell off the rose, however, when a controversial meta-analysis for rosiglitazone changed the discussion from potential CVD efficacy to concerns over CVD safety. Subsequent CVD outcomes studies have not shown increased mortality, but neither have they shown much benefit (PROACTIVE and RECORD trials). The main safety concerns with this class is a fourfold increase in CHF and an acceleration of bone loss resulting in a doubling of fracture rates. TZDs’ best benefits, however, may be prevention of T2DM, which is achieved at lower doses, thereby reducing these safety concerns.19
α-Glucosidase Inhibitors (AGIs)—the Drugs that Get no Respect
Drugs in this class are frequently the butt of jokes and no one takes them seriously. Like Rodney Dangerfield, these drugs ‘don’t get no respect’, which is unfortunate given all of their positive attributes. Their mechanism of action is simple: they block the digestion and absorption of ingested polysaccharides, thus reducing the level of postprandial glucose and insulin excursions. Although the major impact is on postprandial glucose, they also reduce fasting glucose and HbA1c. This class of drugs has many positive features: no hypoglycaemia, consistent loss of body weight and the progression of T2DM is slowed. Blood pressure, triglycerides, inflammatory biomarkers and the development of hypertension are all reduced. Even better, these positive surrogate markers result in positive outcomes as measured by reduced progression of atherosclerosis (vascular intimal medial thickness) and cardiovascular events in an impaired glucose-tolerant (IGT) population (STOP-NIDDM trial). Even though this is a very safe drug class, almost half of the patients will have gastrointestinal complaints. Carbohydrate is usually absorbed in the upper intestine; upon initiation of AGIs, a large portion escapes digestion and is delivered to the colon causing excessive bacterial activity. To avoid this, AGIs should be titrated slowly over several months, giving time for the lower small intestine to acquire the ability to absorb carbohydrates.
AGIs are a good fit with metformin, although both have gastrointestinal side effects. AGIs are particularly effective in mild hyperglycaemia and in correcting postprandial hyperglycaemia. AGIs have CVD outcomes data and are very safe. These drugs should be thought of more often when prescribing antiglycaemics.
Colesevelam: LDL and A1c Lowering—a Match Made in Heaven
The bile-acid binding resin colesevelam was approved for LDL lowering in 2000 and 8 years later for glucose lowering in T2DM. This class of drugs has already been shown to reduce CVD and mortality, although not in a T2DM population.
The mechanism for colesevelam’s antiglycaemic action is unknown, but the alteration in bile acid composition produces two possible antiglycaemic actions: (1) increased delivery of fatty acids to the distal intestine stimulating GLP-1 secretion and (2) activation of hepatic FXR (farnesoid X receptor), which inhibits gluconeogenesis. The combination of increased GLP-1 action and decreased hepatic gluconeogenesis is like combining a DPP4I (dipeptidylpeptidase-4 inhibitor) with metformin (e.g., Janumet).
To date, colesevelam does not have micro- or macrovascular endpoint data, but it does lower both glucose and LDL with negligible hypoglycaemia and weight changes.20 Colesevelam’s LDL-lowering effects are additive with statins. The combination of colesevelam and a statin permits a reduction in the statin dose and risk of rhabdomyolysis and attenuates the rise in triglycerides observed with this class of drugs.
Because it is a bile acid-binding resin, it does not have systemic effects, but the main complaints are gastrointestinal. Recently, a convenient single-dose packet dissolved in a glass of water has replaced the onerous six capsules daily.
Bromocriptine: Born Again
Physicians have been using this drug for many years to treat prolactin-secreting pituitary tumours, Parkinson’s disease and many other off-label uses. Now it has been re-born as a quick-release formulation, Cycloset (0.8 mg tablets, VeroScience), to treat T2CM as an adjunct to diet and exercise.21
This is the only antiglycaemic believed to work through the central nervous system. Obese, insulin-resistant T2DM patients have twofold elevated plasma prolactin levels, indicating a hypothalamic dopamine deficiency. Administered as a single timed morning dose, this centrally acting dopamine D2 receptor agonist acts on circadian neuronal activities within the hypothalamus to reset abnormally elevated prolactin, plasma glucose, triglyceride and free fatty acid levels in fasting and postprandial states in insulin-resistant patients.
Bromocriptine may be used as monotherapy or combined with other oral antiglycaemics; use with insulin has not been studied. The recommended starting dose of bromocriptine is 0.8 mg daily and is increased in 0.8 mg increments weekly until the target range (1.6–4.8 mg) or until maximum tolerance is reached. Doses should be administered once daily within 2 h of waking in the morning and with food to reduce the risk for gastrointestinal tract adverse effects such as nausea.
Adverse events most commonly reported in clinical trials of bromocriptine included nausea, fatigue, vomiting, headache and dizziness. These events lasted a median of 14 days and were more likely to occur during initial titration of the drug. None of the reports of nausea or vomiting were described as serious.
Bromocriptine was the first diabetes drug to be approved under the FDA’s new guidelines requiring clinical trials to demonstrate no increased cardiovascular risk. In a 52 week double-blind, placebo-controlled safety trial (n = 3000), treatment with bromocriptine did not increase the risk for a composite of myocardial infarction, stroke, hospitalization for unstable angina, CHF and revascularization surgery. In fact, the risk of this composite CVD endpoint was reduced (hazard ratio, 0.58; 95% confidence interval, 0.35–0.96). This drug appears also to be a good fit as a second agent with metformin and the positive (but preliminary) CVD data are encouraging.
Incretin Therapies: The New Kid on the Block
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








