Abstract
Tumor involving the spinal cord and column can provide for complex decision-making modalities regarding the appropriate treatment options. The pathologic process, the level of disease, and ultimately the overall health and wishes of the patient and family will determine the appropriate treatment modality. Spinal tumor care should be individualized to the patient, family, and pathology of the disease. It is a multidisciplinary team effort and each member is of equal importance. In this chapter we approach all levels of treatment for the patient from the diagnosis to postoperative care.
Keywords
Spinal cord tumors, Spinal metastatic disease, Spinal tumors, Spinal tumor treatment
Background
Tumors of the spine represent a broad and diverse pathologic process which can present physicians with difficult situations regarding diagnosis and treatment. For the purposes of this chapter, emphasis will be placed on the bony spine and the contents contained within, including meninges, spinal cord, and nerve roots. It is important for physicians to understand the various locations where spinal tumors may arise and the pathologic processes from which they arise. Missed or delayed diagnosis can have a significant impact on quality of life as spinal cord compression is a common cause of pain, loss of mobility, and neurologic deficit.
Tumors are either primary or metastatic. Primary spine tumors arise from the spine or its adjacent tissues (spinal cord or meninges), whereas metastatic tumors arise from distant tissues. Primary tumors of the axial skeleton are rare with an estimated incidence of approximately 2.8–8.5 per 100,000 people per year. Metastatic spinal lesions are a relatively more common entity with approximately 18,000 metastatic lesions alone diagnosed each year; this is not surprising given that 70% of cancer patients have metastatic spinal disease.
It is especially important to recognize symptoms of spinal cord compression because up to 12% of patients with spinal disease will develop symptomatic spinal cord compression; 49% of these patients will have multifocal extradural lesions. With the advent of new surgical approaches and nonsurgical means for managing patients with spinal tumors, it will become increasingly important to be able to identify the early signs and symptoms of spinal lesions and to be able to order the appropriate imaging to aid in expedited diagnosis and treatment. Early signs of disease process should be identified upon patient presentation to clinics or the emergency department, followed by evaluation by orthopedic surgery or neurosurgery. After surgical referral, radiation oncology and medical oncology can make further recommendations at a multidisciplinary tumor board. Rehabilitation services are essential for maximizing postoperative outcomes.
Diagnostics
There are many diagnostic studies available to assist today’s physician with diagnosing a spinal tumor. The physical examination discussed earlier should provide the physician with some guidance as to the first location to image. Care must be taken to not order excessive or unnecessary studies, as patients may not be able to tolerate many examinations.
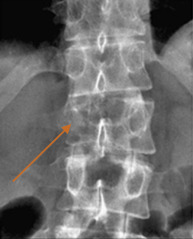
Plain radiographs are a common imaging study and are frequently ordered in patients with back pain. Unfortunately, the site of pain and structure level of compression do not necessarily correlate. This can present a diagnostic issue if the location of the site of compression is not imaged. In one series, the most frequent plain film requested was the lumbar spine, whereas the most common site of compression was the thoracic spine. Nonetheless, if plain films are to be pursued, the “winking owl sign” (radiolucent area obscuring a pedicle, i.e., absent pedicle sign) is the earliest radiographic sign of an osteolytic metastatic lesion ( Fig. 9.1 ). Other signs of spinal metastasis include vertebral body collapse. Plain films have the potential for false-negative results as a tumor must be 1 cm in diameter and there must also be 50% bone mineral loss to be detected; up to 40% of lesions will be undiagnosed.
Computed tomography (CT) scans are another valuable diagnostic tool for the evaluation of patients with suspected spinal pathology. CT scans provide excellent osseous delineation and can identify bony metastatic lesions 6 months earlier than plain films. Sensitivity and specificity of CT scans for identifying histologically confirmed primary spinal malignancies is 66.2% and 99.3%, respectively, with overall diagnostic accuracy of 88.8%. When ordering CT scans, it is important to remember the risks associated with radiation and contrast exposure to the patient.
Magnetic resonance imaging (MRI) is a powerful tool for assessing the spine. The sensitivity and specificity of MRI for identifying histologically confirmed primary spinal malignancies is 98.5% and 98.9%, respectively, with overall diagnostic accuracy of 98.7%. The ability to directly image the spinal cord without presence of bone artifact (found in CT scans) makes MRI the ideal diagnostic for examining the spinal cord. One thing to consider when ordering an MRI is that the acquisition time is significantly longer than a CT scan; patients with pain or claustrophobia may be very uncomfortable and could possibly require sedation. Contrast is also important to help delineate tissues; contrast allergies must be considered prior to obtaining imaging.
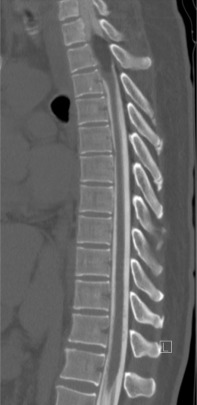
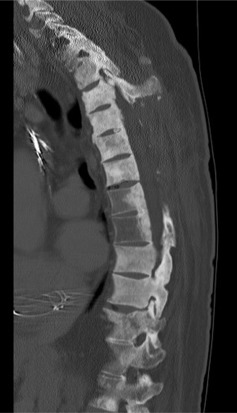
CT myelograms are CT scans obtained after intrathecal administration of contrast ( Fig. 9.2 ). This study is useful in patients who cannot undergo MRI (pacemakers or other non-MRI compatible implants) as it allows the physician to assess osseous integrity and contents within the thecal sac. Intrathecal contrast is administered prior to a traditional CT scan via lumbar puncture and at this time, cerebrospinal fluid may be sent for flow cytometry to assist in diagnosis.
Surgical Management
The widespread availability of MRI has invariably led to earlier diagnosis of spinal tumors. Radiation can be considered in select patients; however, it is generally not first-line therapy. The potential drawbacks of radiation include limited effectiveness of controlling tumor growth, doses of ionizing radiation to the spinal cord/nerve roots, and impaired wound healing, which can present an issue for surgical candidates.
The mainstay of treatment is surgery; however, the extent of surgery and addition of other components such as fusion hardware is a decision that must be made prior to surgery. It is generally accepted that if a patient’s life span is less than 3 months, surgery should not be considered. Surgery is the first option for patients with neurologic deficit and life expectancy greater than 3 months. In general, surgical options are aimed at decompression, i.e., reducing pressure on the spinal cord or nerve roots by removing bone (indirect decompression) or by removing tumor (direct decompression). A laminectomy (removal of the posterior elements ( Fig. 9.3 ) can assist in decompression and tumor debulking when there is posterior involvement. A laminectomy is generally a midline posterior approach which removes the lamina and/or spinous processes; it can be unilateral (hemilaminectomy) or bilateral (laminectomy). Patients are positioned prone on the operating room table when a laminectomy is performed.
A corpectomy/vertebrectomy (removal of the vertebral body) can assist in decompression and tumor debulking when there is anterior tumor involvement. Removal of the anterior vertebral column is more complicated, as a traditional midline posterior approach does not provide enough lateral exposure to access the entire vertebral body. In this instance, modified posterior approaches that provide more posterolateral access may be employed such as a transpedicular approach or lateral extracavitary approach. Once again, prone is the position of choice for posterior approaches. Depending on the level in the thoracic spine, a lateral approach can also be considered when attempting to remove the anterior vertebral column.
Ventral lumbar tumors may also be approached anteriorly. A general surgeon (or anterior access trained orthopedic/neurosurgeon) can gain access to the vertebral body via an anterior retroperitoneal approach, allowing the spine surgeon to decompress, debulk, and instrument the spine via an abdominal incision, where the patient is positioned supine. Similarly, surgically amenable tumors of the spine can be approached via a lateral retroperitoneal transpsoas approach, where a patient is positioned in a lateral decubitus position. It is important to note that when patients have extensive spinal disease with a neurologic deficit that has been present for greater than 48 h, surgery may not be the best option.
In 2005, Patchell et al. compared outcomes of patients with metastatic spinal cord compression who received surgery and radiation versus the previous standard, radiation alone. It was found that 84% of patients who received surgery and radiation were able to walk after treatment versus 54% of patients who received surgery alone. Furthermore, the patients who received surgery and radiation retained the ability to walk for a median of 122 days versus 13 days for those who received radiation alone. From this information, the Patchell criteria was established ( Table 9.1 ).
| Inclusion |
|
| Exclusion |
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree