Abstract
This chapter focuses on cognitive deficits in patients with brain tumors including gliomas, meningiomas, and brain metastasis, as well as their assessment and treatment. Neuropsychologic deficits have been found in multiple cognitive domains, most commonly executive functioning, attention, and memory. Cognitive impairments seem to be mainly multifactorial and not as a consequence of the tumor itself, but the effects of radiation and chemotherapy can also play a major role. Neuropsychologic evaluation is essential to obtain an accurate understanding of a patient’s cognitive and emotional profile and guide therapeutic treatment measures. There is evidence that cognitive remediation and a comprehensive rehabilitation program can benefit patients with brain tumors. Antioxidant medications and medications that target specific cognitive symptoms can also be of significant value.
Key Words
Brain metastasis, Chemo brain, Cognitive deficit, Cognitive remediation, Glioma, Meningioma, Neuropsychologic assessment
Introduction
The average annual mortality in the United States between the years 2010–2014 was 4.33 per 100,000, with over 75,000 deaths being attributed to primary malignant brain or other CNS tumors. Gliomas are the most common primary brain tumor, making up between 70% and 81% of all malignant intracranial primary tumors. The cause is largely unknown. They are classified according to the World Health Organization (WHO) grades 1–4, based on increasing severity of malignancy. Males are more commonly affected than women in a 3:2 ratio.
A grade 4 glioma is known as a glioblastoma and is the most aggressive and accounts for 50%–70% of diffuse gliomas. The average age of diagnosis is 60 years. Anaplastic gliomas are grade 3 lesions including astrocytomas, oligodendrogliomas, or mixed gliomas. They make up 15%–25% of primary brain tumors and usually present around 45 years of age. These higher grade gliomas have been found to present with focal neurologic deficits and cognitive changes of up to 3 months duration and in some cases, headaches of up to 1 month duration.
The remaining 15%–25% of gliomas are low grade—grade 2 and grade 1 gliomas. Grade 2 gliomas include oligodendrogliomas and oligoastrocytomas. Astrocytomas can be grade 1 or grade 2 lesions. These tumors typically present with seizures between 30–45 years of age. Oligodendrogliomas tend to have a median survival of 10–15 years from diagnosis, and astrocytomas and oligoastrocytomas have a median survival of 6 years ( Table 6.1 ).
| WHO Grading | Histologic Classification | Average Age of Presentation | 5-year Survival Rate (%) |
|---|---|---|---|
| I | Pilocytic astrocytoma | 20–30 | 95 |
| II | Diffuse astrocytoma | 30–45 | 50 |
| Oligoastrocytoma | 60 | ||
| Oligodendroglioma | 70 | ||
| III | Anaplastic astrocytoma | 45 | 50–60 |
| Anaplastic oligodendroglioma | |||
| Oligoastrocytoma | |||
| IV | Glioblastoma | 60 | 5 |
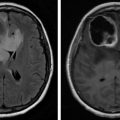
Patients suspected of intracranial lesions should be assessed with MRI, including T1-weighted spin echo, T2 fluid-attenuated inversion recovery (FLAIR), and gadolinium. Lesions suggestive of tumor will have hyposignal on T1 and hypersignal on T2. Lesion biopsy will allow for definitive diagnosis.
Prognosis is based on phenotype and tumor grading. Favorable prognostic factors include younger age, complete surgical resection of tumor, good functional performance status, and good cognition. Management includes steroid treatment in a tapering dose for edema control and antiepileptics for seizure management or prophylaxis. Levetiracetam is usually the antiepileptic of choice as it has a low likelihood to have drug interactions with chemotherapy medications, good tolerability, relatively rapid titration, and intravenous availability. Usually surgical resection of tumor is followed by radiation therapy typically starting 3–4 weeks post operation, once the surgical bed has healed adequately to prevent necrosis from radiation. Ideally, chemotherapy is started shortly after radiation therapy in patients who can tolerate both. The ability to tolerate concurrent chemotherapy and radiation predicts a more favorable prognosis.
Meningiomas, or tumors of CNS dural coverings, have long been the most common nonglial intracranial tumor, accounting for a little over 36% of all such tumors, with an incidence of about 8.3 per 100,000. First described in 1922 by Harvey Cushing, meningiomas have, as a majority, been found to be benign (WHO grade 1) but are typically diagnosed at an older age, with the median age of diagnosis being 66 years old. Furthermore, meningiomas tend to occur with greater frequency in those with certain conditions, such as neurofibromatosis type 2 and multiple endocrine neoplasia type 1. Currently, the WHO criteria has three grades for meningiomas: grade 1 meningiomas are benign, grade 2 meningiomas are atypical, and grade 3 meningiomas are malignant ( Table 6.2 ).
| WHO grade 1—Benign | WHO Grade 2—Atypical | WHO Grade 3—Malignant |
|---|---|---|
| Meningothelial | Chordoid | Papillary |
| Fibrous | Clear cell | Rhabdoid |
| Transitional/Mixed | Atypical | Anaplastic |
| Psammomatous | ||
| Angiomatous | ||
| Microcystic | ||
| Secretory | ||
| Lymphoplasmacyte-rich | ||
| Metaplastic |
Surgery is necessary for establishing a definitive diagnosis and removal of the tumor, but gross total resection (GTR) is only achieved about 50% of the time. Depending on the extent of resection of the meningioma, the Simpson criteria has been able to better predict the risk of tumor recurrence. But there still remains some uncertainty, and hence there is some variability in treatment options. Available treatments include surgical resection with recurrence being based on the Simpson classification Table 6.3 , single-session stereotactic radiosurgery, hypofractionated stereotactic radiation therapy, and conventionally fractionated external beam radiation therapy.
| Grade | Definition | Recurrence Rate |
|---|---|---|
| 1 | Gross total resection (GTR) of tumor, dural attachments, and abnormal bone | 9 |
| 2 | GTR of tumor, coagulation of dural attachments | 19 |
| 3 | GTR of tumor without resection or coagulation of dural attachments or extradural extensions | 29 |
| 4 | Partial tumor resection | 44 |
| 5 | Simple decompression (biopsy) |
The primary objective of surgery for meningiomas is that which is classified as Simpson grades 1–3. In benign meningiomas, GTR is considered definite therapy. Simpson grades 4 and 5 or subtotal resections, on the other hand, carry a substantially higher rate of progression, even with benign meningiomas. Moreover, other treatment options such as radiation therapy are typically used as an adjunct therapy to subtotal resection, treatment for tumor recurrence, for tumors in surgically inaccessible areas, or tumors of high-grade histology. On the other hand, chemotherapy has a limited role in meningiomas and hence is rarely used.
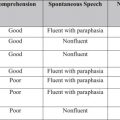
Clinical presentation in brain tumors can be very diverse, involving any conceivable neurologic or neuropsychologic sign or symptom by virtue of their localization, tumor type, and growth characteristics. In turn, the clinical presentation interacts with different patient characteristics including age, cerebral lateralization, premorbid abilities and skills, and environmental demands. Slowly growing tumors are often associated with an insidious onset, and subtle progression of symptoms not unlike it is observed in neurodegenerative disorders such as dementia. If cognitive symptoms are the earliest signs of a tumor, the impairments are usually severe enough to interfere with the patient’s daily activities. Unawareness, which may accompany the impairments resulting from a brain tumor, may also delay its identification. While relatives and friends may misattribute the cognitive, emotional, and personality changes to other benign causes, it is common for them to retrospectively identify subtle tumor signs that were present before the condition was suspected. Initial presentation may involve a single breakthrough event such as a seizure or an episode of altered mental status or aphasic/dysphasic speech. At times, prior to the emergence of cognitive symptoms, focal, noncognitive neurologic signs such as numbness or tingling, or signs of increased intracranial pressure—headaches, nausea, vomiting, drowsiness, and visual abnormalities—may be present.
Inpatient and outpatient rehabilitation therapy to address functional impairments as a result of weakness, mobility, and ADL impairment and cognitive deficits due to the effects of the tumor itself or the side effects of cancer treatment is of benefit. Patients and their families should also be referred to psychologic counseling and support to address coping strategies to address the psychologic stress and social impact of this progressive disease.
Tumor-Related Cognitive Decline
Early in the course of diagnosis, patients with gliomas, meningiomas, and cerebral metastasis often exhibit neurocognitive deficits that impact general functioning and overall quality of life. In general, for all of these tumor types, deficits have been noted in multiple cognitive domains. Even patients considered to be in good neurologic status at diagnosis were shown to have significant deficits in neuropsychologic assessments of processing speed, executive functioning, and memory. Larger tumor volume, tumors affecting the frontal lobe, and left cortical lesions seem to be associated with greater neurocognitive dysfunction. It appears that in patients with gliomas, executive functioning tends to be the most commonly affected. Attention and memory have also been noted to be frequently impaired. These cognitive deficits have been observed both prior to and after surgical resection. Meningioma tumors tend to more frequently affect frontal lobe functions of attention, executive functioning, and memory. Patients with cerebral metastatic lesions are most commonly affected by memory deficits.
Gliomas
There are a handful of studies addressing cognitive deficits in glioma early in diagnosis. In a neuropsychologic assessment of patients with both low- and high-grade gliomas prior to the initiation of any treatment, deficits were found in multiple cognitive domains, most commonly executive functioning. Greater than 60% of patients had impairment in at least one cognitive domain of executive functioning, attention, memory, language, visuospatial functioning, and processing speed. Patients with high-grade glioma are affected with a greater degree of cognitive impairment compared with patients with low-grade glioma.
Neuropsychologic evaluation of a group of patients with low-grade gliomas found 60% of these patients had cognitive deficits, mainly in executive functioning but also in domains of attention and memory. Two-third had subjective complaints of fatigue or attentional disturbances that had been progressively increasing over the past 6–12 months. There was a positive correlation between subjective complaints and impairments on neuropsychologic testing.
Another study showed 1/3 of patients with high-grade glioma exhibited cognitive decline after treatment with chemotherapy and radiation. However, the overwhelming majority of these patients had progression of their tumor within 4 months. This finding suggests that cognitive decline often appears to be related to tumor progression rather than treatment. It has also been shown that there is a significant correlation between deficits on the neuropsychologic tests of the Controlled Oral Word Association (COWA), which assesses executive functioning, and Trails Making Test B, which measures attention, with increased mortality. A significant, but less prominent, correlation was also observed in deficits on the executive task of similarities and the attention task of digit span on chance of survival. There was no association between tumor laterality and survival.
It is noteworthy to mention that much of the literature on this topic is limited by small study sample sizes and heterogeneity of assessment measures used. Almost all patients had cortical lesions. Overall, a sizable percentage of these patients exhibited some degree of cognitive impairment on neuropsychologic evaluation.
Meningioma
Most patients with meningioma demonstrate cognitive deficits in several domains, particularly frontal lobe functions. A systematic review assessing cognitive deficits in patients with meningioma (mostly affecting the cortex) before and/or just after treatment found that in general, patients with meningioma have cognitive deficits mainly in attention, memory, and executive functioning. Typically post resection most studies found improvement in cognition, but patients still had deficits compared with healthy controls. Most studies did not find significant association of tumor laterality and cognitive impairment. A couple studies suggested that right-sided lesions tend to improve more post resection compared with left-sided lesions.
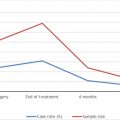
Skull base meningioma tumors can also affect cognition. A prospective study assessing neuropsychologic measures in patients with skull base meningioma prior to and at 3–5 months and 9–12 month periods after surgical resection showed about 10%–15% of patients showed decline in long-term verbal memory, working memory, and processing speed within 3 months after surgical resection, but only 5%–10% of patients showed long-term impairments persisting at 12 months. No significant effect was noted for measures of anxiety and depression. Authors did not speculate or comment on factors associated with a decline in neurocognitive status. Anterior skull base meningiomas affecting the ventromedial prefrontal cortex exhibited significant decline in behavioral adaptive functioning, but do not affect basic cognitive functioning.
Brain Metastasis
Common primary tumors with metastasis to the brain include lung, breast, melanoma, and colon cancer. Compared with glioma and meningioma, there is limited literature regarding cognitive deficits related to brain metastasis. Overall, patients with brain metastasis were significantly impaired in most cognitive domains compared with controls. Memory deficits were found to be the most prominent. Language, executive functioning, and fine motor dexterity were also found to be significantly impacted in a number of patients. Attention was found to be the least affected. Cognitive deficits would be expected based on the region of brain affected and the extent and size of metastatic lesions in the brain.
Treatment-Related Cognitive Impairments
Overall, the treatments for malignancies have improved, allowing for individuals with cancer to better fight off their disease process. With increased survivorship among patients with malignancies comes the long-term sequelae of the treatments we provide them. In particular, individuals with brain malignancies experience cognitive impairments related to treatments such as radiation therapy, chemotherapy, and surgery. Much of the improvement in treating brain tumors has come from this increased aggressiveness in attacking the tumor, combining multiple treatment options. The cognitive impairments as a result of these treatments, however, are a feature that may limit a patient’s functional recovery, as they typically affect the processes of learning, concentration, memory, spatial information processing, reasoning, attention, and processing speed. There has been some variability in the literature as to the effect that tumor size and grade actually have on cognitive impairments. It is therefore important to identify and address other potential causes of these impairments, helping to minimize these effects, especially since cognitive function has been shown to be a sensitive independent predictor of survival.
Neurosurgery
The choice of surgical procedures (i.e., craniotomy, stereotactic biopsy, image-guided biopsies) and the location of the tumor will determine the impact on cognition. Also, the extent of surgical intervention will determine the risk of cognitive deficit. Many intracranial tumors are susceptible to be treated by means of neurosurgical resection. Even large meningiomas can frequently be excised completely. Surgery for metastatic tumors is continuously evolving and has benefitted from improved neuroimaging resulting in improvement in intraoperative targeting, anesthetic techniques, and survival rates.
Complete surgical removal of an intracranial tumor typically results in a relatively stable and focal chronic postsurgical neuropsychologic profile during the recovery period. In the case of space-occupying tumors from the cranium, their removal frequently results in neuropsychologic improvement compared with the patient’s presurgical status.
Radiation Therapy
Radiation therapy has proven to be a curative therapeutic tool in the treatment of cancer, but it has also been shown to have a negative impact on quality of life as well as neurocognitive function. The effect of a brain tumor itself on a patient’s function and cognitive status is dependent on location, but radiation-induced brain injury is a dynamic process affecting all cell types: endothelial and oligodendroglial cells, astrocytes, microglia, neurons, and neuronal stem cells. The effects of radiation therapy are divided into acute, early, delayed, and late delayed processes, with late delayed effects believed to be the cause of neurologic consequences. Delayed effects may present up to 20 years after treatment. Characteristics of radiation therapy that contribute to the risk of radiation-induced brain injury include total dose, dose per fraction (higher risk with greater than 2 Gy dose per fraction), total time and volume, radiation quality and volume, hyperfractionated schedules, shorter overall treatment time, presence of comorbid vascular risk factors, and adjunctive therapy. Radiation therapy causes both demyelinating and axonal damage, which are hallmarks of brain white matter damage. The proposed mechanism is believed to be by damaging oligodendrocytes and disrupting cerebral vascular endothelial cells leading to coagulative necrosis, vessel wall thickening, and focal mineralization, affecting brain white matter more than brain gray matter. Radiation to larger volumes of brain and higher doses have also been shown to produce higher grade lesions on magnetic resonance imaging, and the amount of demyelination and damage to the white matter track integrity correlates with the extent of cognitive impairment. Additionally, radiation therapy is generally not given as a monotherapy, rather combined with chemotherapy targeted against the tumor as well.
Partial brain radiotherapy results in variable effects across patients, with prevalent impairment of memory and processing speed. Extent of impairment of verbal versus visual memory may vary from the early to the last postradiation phases. However, the initial neuropsychologic profiles do not appear to accurately predict profiles after 3 years.
Limited field radiation appears to have less delayed consequences than those seen in whole brain radiation. Most patients appear to have minimal or no cognitive deterioration during the first several years, with some variability among cases. Increasingly more used in the treatment of several types of brain tumors, stereotactic radiosurgery, has a more focused effect and therefore limits the amount of radiation delivered to normal tissue and the contingent neurocognitive deficits.
Chemotherapy
Typically speaking, radiation therapy is localized to a certain region of the body; however, chemotherapy has a more systemic effect. There are a vast number of chemotherapeutic agents available for cancer treatment, each affecting an individual’s body differently but also carrying their own side effect profile. White matter in the brain is significantly vulnerable to effects of chemotherapy, with onset of identifiable changes possibly delayed for months. A wide range of incidence for cognitive impairments in those receiving chemotherapy has been reported, but of more recent it is believed to be greater than 70% and typically manifesting as concentration impairments, memory loss, reasoning, and attention deficits. These deficits are typically referred to as “chemobrain.” This is not to say that the high incidence of cognitive impairments in those being treated with chemotherapy is solely due to the medications themselves, but may be a contributing factor in addition to the tumor itself.
Most chemotherapeutic agents used do not even cross the blood-brain barrier, with the exception of 5-Fluorouracil, methotrexate, and a few others which were found in low levels including cisplatin, carmustine, and paclitaxel. Even so these chemotherapeutic agents are believed to increase overall oxidative stress resulting in cognitive impairments. Chemotherapy produces a wide variety of cognitive impairments, most of which are dose dependent. Furthermore, as many cancer treatment plans are multifaceted, it has been shown that the toxicity of radiation, as mentioned earlier, is likely to be synergistic with the toxicity of chemotherapies, further exacerbating these symptoms. Historically, it was believed that these cognitive impairments were related to psychologic factors such as depression, anxiety, or other cancer-related side effects such as fatigue. Furthermore, prophylactic medications such as antiepileptic medications may exacerbate fatigue and lethargy. Steroids are used to reduce edema and may cause insomnia, anxiety, or emotional lability. Both of these types of medications may exacerbate cognitive impairments. As we, medical professionals, look to be more aggressive in treating an individual’s cancer, we must remember the potential for side effects related to the treatments we are providing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree