Introduction
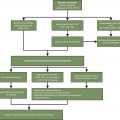
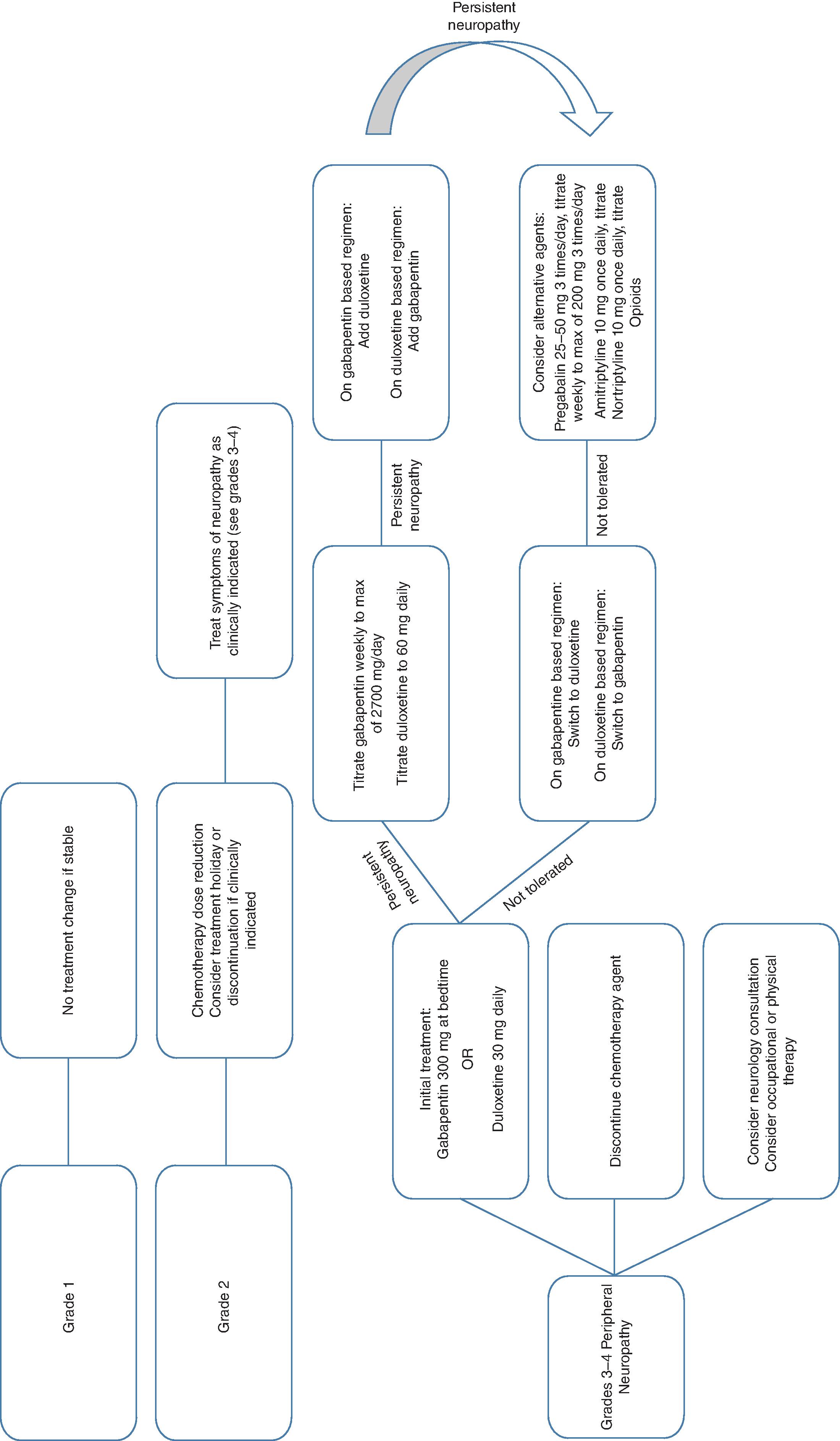
Chemotherapy-related neurotoxicity is a frequently observed side effect that has become more prevalent with the increasing number of long-term cancer survivors. These toxicities can be peripheral or central and can range from minor cognitive issues to encephalopathy or dementia. Toxicities are often dose-limiting, resulting in dose reduction or treatment discontinuation, potentially compromising the therapeutic efficacy. This chapter will discuss the incidence, mechanism, symptoms, and management of chemotherapy-related neurotoxicity with a focus on the more common chemotherapeutic agents. The Common Terminology Criteria for Adverse Events (CTCAE) is widely used in the evaluation of chemotherapy-induced peripheral neuropathy. ( Table 7.1 ) Fig. 7.1 illustrates an algorithm for the evaluation and treatment of chemotherapy-related peripheral neuropathy.
| Grade | Definition |
|---|---|
| 1 | Asymptomatic; clinical or diagnostic observations only |
| 2 | Moderate symptoms; limiting instrumental ADL |
| 3 | Severe symptoms; limiting self-care ADL |
| 4 | Life-threatening consequences; urgent intervention indicated |
Chemotherapy-Related Neurotoxicity
- ■
Taxane chemotherapy: Taxane-induced peripheral neuropathy is a class effect of toxicity, but is more commonly reported with paclitaxel (57%–83%) compared to docetaxel (11%–64%). Although the exact mechanism is not well understood, the most widely accepted hypothesis of taxane-induced neuropathy is the disruption of the axonal microtubule structure. Taxanes inhibit tubulin depolymerization and disrupt the formation of microtubules, including the axon of neurons. Intact microtubules are essential for axonal transport and neuronal survival, and alteration of their structure can lead to peripheral neuropathy.
- ■
Presentation: Taxane-induced neuropathy generally presents with sensory loss in the hands and/or feet in a glove-and-stocking type of distribution. Symptoms include numbness, paresthesias, dysesthesias, unsteadiness, and loss of balance, which can impede quality of life and functional status. Motor impairment is unusual, but muscle aches and loss of strength have occurred. ,
- ■
Relationship to dose: Taxane-induced peripheral neuropathy can occur with high cumulative doses, with onset of symptoms typically occurring with doses greater than 300 mg/m 2 of paclitaxel. Severe symptoms of peripheral neuropathy are generally not seen until doses exceed 400 mg/m 2 of docetaxel. High single doses of paclitaxel are also associated with an increased risk—up to 75% of patients who receive 175 mg/m 2 once weekly develop severe peripheral neuropathy. Studies have had conflicting observations regarding the optimal dosing interval of paclitaxel to minimize neurotoxicity. The infusion rate has also been shown to be a risk factor for paclitaxel-induced neuropathy, with shorter infusions (1 or 3 hours) associated with higher rates of peripheral neuropathy when compared with 24-hour infusions. ,
- ■
Treatment: There are no known prophylactic agents and treatment options are limited. Table 7.1 describes treatment options for neuropathy. Most patients experience at least a partial resolution of symptoms within 3 to 6 months of drug discontinuation. Many patients, however, do not experience complete symptom resolution. Up to 40% of patients can have symptoms years after treatment completion. Several agents have been evaluated for the treatment of peripheral neuropathy ( Table 7.2 ).
TABLE 7.2
Agent, Dose, and Duration of Therapies for Chemotherapy-Related Peripheral Neuropathy
Drug
Dose
Notes
Duloxetine a
Initial: 30 mg once daily
Escalation: 30 mg/week to target 60 mg
•Serotonin syndrome monitoring required with concomitant medications•
Risk of withdrawal syndrome when discontinued abruptly•
Similar dosing to treat mood disorders
Gabapentin
Initial: 300 mg once daily
Escalation: 300 mg/week to target dose of 2700 mg/day in divided doses
•Initial dosing at bedtime to decrease sedation•
Adjust in renal insufficiency
Lamotrigine
Initial: 25 mg once daily
Escalation: 25 mg twice daily, 50 mg twice daily, 100 mg twice daily, 150 mg twice daily every 2 weeks
•Slow titration to decrease risk of Steven Johnson Syndrome (SJS)
Nortriptyline
Initial: 10–25 mg once daily
Escalation: Weekly to target of 50–100 mg once daily
•Serotonin syndrome monitoring required with concomitant medications•
Initial dosing at bedtime to decrease sedation•
Higher doses than used in peripheral neuropathy normally required for treatment mood disorders
Amitriptyline
Starting: 10 mg once daily
Escalation: 10 mg/week to target 50 mg
•Serotonin syndrome monitoring required with concomitant medications•
Initial dosing at bedtime to decrease sedation•
Higher doses than used in peripheral neuropathy normally required for treatment mood disorders
a Several agents have been studied in randomized trials but only duloxetine had positive outcomes when used as a preventive measure.
- ■
- ■
Platinum based chemotherapy
- ■
Cisplatin: Cisplatin can cause peripheral neuropathy, with symptoms such as numbness, paresthesias, and dysesthesias, especially with cumulative doses.
- ■
Mechanism: The development of platinum-induced peripheral neuropathy is attributed to damage of the dorsal root ganglion (DRG) caused by the formation of intrastrand adducts and interstrand crosslinks.
- ■
Relationship to dose: Symptoms are reported at doses greater than 300 mg/m 2 of cisplatin, and 90% of patients have evidence of neuropathy at cumulative doses of greater than 600 mg/m 2 . Although peripheral neuropathy caused by other chemotherapeutic agents normally improves with treatment completion, 30% of patients with platinum-induced neuropathy can experience a coasting effect where neuropathy worsens several months after drug discontinuation, before any improvement is noted. Symptom improvement is seen in the majority of patients but in several long-term survival studies, 10% to 30% of patients reported mild symptoms of neuropathy up to 15 years after treatment. , ,
- ■
Encephalopathy: Cisplatin has limited blood–brain barrier penetration and reports of encephalopathy are rare with intravenous administration. Neurotoxicity, however, has occurred after intraarterial cisplatin administration, with headaches, encephalopathy, seizures, and cortical blindness all having been reported.
- ■
Ototoxicity: Cisplatin-induced ototoxicity with high-frequency hearing loss has been described in patients receiving cisplatin. This toxicity is commonly attributed to sensory hair cell death in the cochlea, and develops with increasing cumulative doses. The incidence varies, with reports ranging from 17% to 80% in clinical trials. The primary risk factor is cumulative dosing of cisplatin. Other risk factors include younger age, concurrent radiation to the cochlea or cranial nerves, and renal dysfunction. There are no known effective treatment or prophylactic options for cisplatin-induced ototoxicity in adults. Prophylaxis with vitamin E and amifostine have failed to show benefit in randomized controlled trials and are not recommended. Sodium thiosulfate has been studied as an otoprotective agent that acts by inactivating cisplatin and preventing sensory hair cell death in the cochlea. Historically, it has not been recommended as a standard of care due to concern that the inactivation of cisplatin may negate the efficacy of the chemotherapy treatment. A trial in 109 children younger than 18 years suggested that there is no difference in treatment efficacy if sodium thiosulfate administration is delayed by several hours. In this trial, 57 children received cisplatin 80 mg/m 2 plus sodium thiosulfate, and 52 children received cisplatin alone. Sodium thiosulfate 20 g/m 2 was given 6 hours after cisplatin administration. Grade 1 hearing loss or greater was reported in 33% of patients in the sodium thiosulfate group versus 65% in the cisplatin alone group ( P = .002). Three-year event free survival and overall survival were similar in both groups. Further studies in the adult population are needed to determine sodium thiosulfate’s place in the prevention of ototoxicity. ,
- ■
- ■
Carboplatin: Carboplatin is a second-generation platinum agent that is less neurotoxic than cisplatin. Peripheral neuropathy and ototoxicity have been reported, but generally only with high doses. ,
- ■
Oxaliplatin: Oxaliplatin produces two types of neurotoxicity, an acute cold-triggered neurotoxicity that is usually transient and a chronic dose-limiting peripheral neuropathy.
- ■
Acute cold-triggered neuropathy occurs in 90% of people receiving oxaliplatin and is characterized by distal paresthesias, jaw pain, hand and foot muscle contractions, and dysesthesias. It is exacerbated by cold temperatures and normally reverses within 1 week, although unresolved symptoms between treatments can occur with increasing numbers of cycles. Although the mechanism of oxaliplatin-induced acute neurotoxicity is not fully understood, the leading hypothesis is that oxalate, an oxaliplatin metabolite, chelates calcium causing changes to voltage-dependent sodium channels and hyperexcitability of peripheral nerves.
- ■
Chronic dose-limiting peripheral neuropathy is attributed to damage of the DRG caused by the formation of intrastrand adducts and interstrand crosslinks, which is similar to cisplatin, except that cisplatin produces three times more adducts in the DRG and is associated with more neurotoxicity then oxaliplatin. Peripheral neuropathy is seen in 45% of patients with cumulative doses of oxaliplatin. Like other platinum agents, patients can experience a coasting effect where neuropathy temporarily worsens several months after drug discontinuation. However, several long-term studies have reported that neuropathy is reversible in the majority of patients, with only 13% reporting mild symptoms 4 years after treatment with oxaliplatin. ,
- ■
Preventative options are limited for oxaliplatin-induced neuropathy. Lengthening the infusion duration from 2 to 6 hours has failed to decrease the incidence of neurotoxicity. Prophylaxis with vitamin E, acetyl-l-carnitine, glutamine, α-lipoic acid, magnesium, calcium, and sodium thiosulfate have not shown benefit in randomized controlled trials and are thus not recommended. , The only proven option to reduce the incidence of neuropathy comes from the OPTIMOX-1 and CONcePT trials. These trials reported that alternating non–oxaliplatin-containing regimens in patients receiving palliative chemotherapy for metastatic colorectal cancer may decrease the risk of severe neuropathy while not compromising efficacy. In certain clinical situations, it may be appropriate to substitute or hold oxaliplatin until the progression of disease in patients responding to oxaliplatin-based therapy to prevent the development of neurotoxicity. ,
- ■
- ■
Vinca alkaloids: Vinca alkaloids are associated with a variety of neurological toxicities, including peripheral neuropathy and autonomic neuropathies. Of the vinca alkaloids, vincristine is the most neurotoxic compared to vinblastine and vinorelbine. Vincristine also has a dose-limiting toxicity of axonal neuropathy. Vincristine binds to tubulin to prevent the formation of microtubules, altering their structure and interfering with axonal transport. This damage results in paresthesias in the fingers and toes, gait disturbances, loss of ankle reflexes, distal weakness, and foot drop in 57% of patients treated.
- ■
Risk factors: Several risk factors increase the incidence and severity of neuropathy. High single doses or cumulative doses between 30 and 50 mg are correlated with increasing neuropathy. Due to this, most treatment regimens cap a single dose of vincristine at 2 mg. Those with underlying neuropathy disorders are at higher risk and vincristine is contraindicated in patients with Charcot-Marie-Tooth disease. Other risk factors include hepatic impairment, older age, concomitant radiation, and administration with CYP3A4 inhibitor medications. ,
- ■
Prophylaxis: There are no known prophylactic options for vincristine-induced neuropathy. Studies are inconclusive on whether neurotoxicity decreases when vincristine is administered as a continuous infusion instead of a bolus and this is not routinely recommended. An adrenocorticotropic hormone (ACTH) analog called Org 2766 was found to prevent cisplatin- and vincristine-based neuropathy in several small trials but failed to show benefit in larger trials. Small trials suggested a decrease in the incidence of neuropathy using glutamic acid, but larger trials are still investigating the long-term outcomes.
- ■
Outcomes: Although some patients experience temporary worsening of their neuropathy several months after treatment discontinuation, most long-term studies report resolution of symptoms several months to years later. See Table 7.2 for preventive strategies for peripheral neuropathy.
- ■
Autonomic neuropathies , such as constipation or abdominal pain, occur in up to 40% of patients, requiring the initiation of appropriate bowel regimens in all patients receiving vinca alkaloids. Rarely, this can lead to paralytic ileus or megacolon.
- ■
Focal mononeuropathies that involve cranial nerves may occur with vincristine, and rare reports of facial atrophy, ptosis, hearing loss, and retinal damage have occurred.
- ■
CNS toxicity is uncommon with vincristine unless an overdose has occurred. Rarely inappropriate secretion of antidiuretic hormone (SIADH) may develop, resulting in hyponatremia, confusion, and seizures.
- ■
- ■
Antimetabolites
- ■
Methotrexate: The development of acute, subacute, and delayed neurotoxicity with methotrexate (MTX) is dependent on the dose and route of administration.
- ■
Mechanism: The mechanism of action is uncertain but is likely caused by inhibition of folate and methionine metabolism, resulting in disruption of folate homeostasis in the central nervous system (CNS). ,
- ■
Aseptic meningitis has been described in 10% of patients who receive intrathecal MTX. Literature reports up to 50% of patients have been affected, but this rate has decreased significantly due to the introduction of microfiltration of MTX. Symptoms, such as headaches, stiff neck, fevers, nausea/vomiting, and back pain occur 2 to 4 hours after intrathecal MTX administration and resolve within 72 hours. These symptoms are normally self-limiting and do not require treatment. If additional doses are required, administering intrathecal MTX with intrathecal hydrocortisone has been reported to be protective against some symptoms of aseptic meningitis. ,
- ■
Transverse myelopathy has been rarely reported after intrathecal MTX administrations. Symptoms begin with back or leg pain and can rapidly progress to paraplegia and sensory loss. The onset is between 1 and 48 hours, although there are reports several weeks after administration. Although there is commonly improvement in symptoms, recovery rates vary, and most patients do not return to baseline. There is no standard of care treatment, but a case report on using S-adenosylmethionine (SAM) 200 mg three times daily, folinate 20 mg four times daily, cyanocobalamin 100 µg once daily, and methionine 5 g daily recorded complete resolution of symptoms in Europe. However, further studies are needed before this treatment can be routinely recommended.
- ■
Subacute neurotoxicity can manifest as encephalopathy, seizures, aphasia, or stroke-like symptoms. The median onset is 2 to 10 days after systemic high-dose MTX administration. Symptoms normally resolve within 72 hours and do not require treatment.
- ■
Leukoencephalopathy can occur months to years after therapy and is often considered the most significant delayed toxicity of MTX, with trials reporting an incidence as high as 20%. Leukoencephalopathy most commonly manifests as progressive cognitive dysfunction that can result in dementia, somnolence, and seizures. Symptoms can stabilize or improve after MTX discontinuation, but the clinical course is variable. The mechanism is unknown, but it often occurs after repeated doses of IV high-dose MTX with or without recent cranial radiation. With the exception of avoiding concurrent cranial radiation and high-dose MTX when possible, there are no treatment or prophylaxis strategies available to prevent leukoencephalopathy.
- ■
Monitoring of methotrexate: MTX is almost exclusively excreted in the urine and can precipitate in acidic urine (pH < 7), thus maintaining alkaline urine is important to prevent toxicity. Furthermore, the risk of these toxicities is influenced by clearance of the drug. For this reason, MTX levels should be monitored at least daily until plasma levels drop below 0.1 µM. It is generally expected to reach these levels 72 hours after high-dose MTX administration. Leucovorin, a form of folic acid, can be used for leucovorin rescue after high dose MTX administration.
- ■
- ■
Fluorouracil: Fluorouracil crosses the blood–brain barrier and there are rare reports of cerebellar ataxia, extrapyramidal syndromes, nystagmus, and dysarthria weeks to months after treatment. Neurotoxicity can occur much more commonly in patients who are dihydropyrimidine dehydrogenase (DPD) deficient and are unable to metabolize fluorouracil or capecitabine. Despite this, routine testing for DPD deficiency prior to treatment is not recommended.
- ■
Fludarabine: Neurotoxicity caused by fludarabine at conventional doses is uncommon, with most large trials reporting an incidence of less than 1%. In these rare cases, symptoms are normally mild, consisting of headaches, somnolence, and confusion, and resolve after treatment cessation. At doses greater than 90 mg/m 2 /day, more severe encephalopathy, cortical blindness, seizures, and ataxia can occur in up to 36% of patients. There are no known treatment or preventive measures. ,
- ■
Cytarabine: High doses of cytarabine greater than 1 g/m 2 /day have been reported to cause acute cerebellar syndrome, with a reported incidence up to 25% in patients who receive in excess of 3 g/m 2 /day. Symptoms occur within 2 to 5 days of treatment and can range from somnolence and ataxia to dysarthria and nystagmus. In rare cases, seizures have been reported. Patients are at higher risk if they have received cumulative doses greater than 36 g, have renal dysfunction, hepatic dysfunction, elevated alkaline phosphatase levels, or are older. There are no known treatment or preventive measures, but most patients have complete recovery within 2 weeks when cytarabine is discontinued immediately on symptom onset. , , Similar to intrathecal MTX, intrathecal cytarabine can rarely cause aseptic meningitis and myelopathy.
- ■
Nelarabine: Central and peripheral neurotoxicity have been reported as dose-limiting side effects in phase I and II trials, with 65% of patients experiencing some form of neurotoxicity. The most common manifestations included fatigue, somnolence, and confusion that occur within the first few weeks of treatment initiation. More severe toxicities, such as seizures and hallucinations, have been reported infrequently. With the exception of peripheral neuropathy, there does not appear to be a correlation between dose and toxicity severity. , , Although peripheral neuropathy has been reported after the first course of treatment in some patients, it usually developed after cumulative cycles of therapy. There are no known prophylactic agents for nelarabine-induced neurotoxicity, and treatment options are limited to symptom management; 90% of patients experienced resolution of their symptoms with drug discontinuation. See Table 7.2 for options for peripheral neuropathy.
- ■
Ifosfamide: Ifosfamide-induced encephalopathy has been reported in 10% to 30% of patients receiving ifosfamide within 12 to 146 hours of administration, and normally resolves within 48 to 72 hours of onset.
- ■
Symptoms: The most common manifestations include confusion, decreased level of arousal, stupor, and somnolence. Rarely, extrapyramidal symptoms, seizures, hallucinations, personality changes, and comas have been reported.
- ■
Mechanism: There are several proposed mechanisms of ifosfamide-induced neurotoxicity. Ifosfamide is a prodrug that is activated by CYP3A4 into 2- and 3-dichloroethylifosfamide and chloroacetylaldehyde. Chloroacetylaldehyde causes the depletion of glutathione which has been linked to ifosfamide neurotoxicity. Alternatively, subsequent studies linked chloroacetylaldehyde’s effect on inhibition of long-chain fatty acid metabolism to ifosfamide encephalopathy.
- ■
Risk factors: Higher incidences of encephalopathy have been reported with bolus or short infusion administrations, renal impairment, low serum albumin levels, concomitant aprepitant, hepatic impairment, prior ifosfamide-induced encephalopathy, prior cisplatin administration, and poor performance status.
- ■
Prophylaxis: There are limited data on the use of methylene blue as prophylaxis for encephalopathy and use based on institutional preference. Methylene blue is believed to act as an alternative electron acceptor that inhibits the transformation of chloroethylamine into chloroacetylaldehyde and stimulates long-chain fatty acid oxidation. Several case reports and small trials have looked at its use for prophylaxis. Based on that data, methylene blue, 50 mg every 6 hours, can be considered for the duration of the ifosfamide infusion if patients have risk factors such as previous ifosfamide neurotoxicity, renal dysfunction, or low serum albumin levels. ,
- ■
Ifosfamide-induced encephalopathy is self-limiting and resolves within 72 hours after drug discontinuation. There are case reports using methylene blue, thiamine 100 mg every 4 hours, or dexmedetomidine for treatment.
- ■
Chemotherapy-Induced Neurocognitive Deficit (Chemo-Brain)
Improvements in cancer treatment have resulted in an increasing number of cancer survivors worldwide with an emphasis on late effects, including treatment-related cognitive impairment, colloquially known as chemo-brain .
- ■
Presentation: Symptoms include difficulties with word finding, memory, concentration, processing speed, and multitasking. , This can occur during treatment or shortly after treatment discontinuation, with variable recoveries. Some report cognitive recovery within 6 months to 2 years, whereas others report lifelong difficulties. The incidence differs among malignancies, with reports between 15% and 60% in trials. There are no definitive risk factors identified in clinical trials but most report baseline fatigue, depression, and decreased cognitive reserve to increase risk.
- ■
Mechanism: Although the mechanism is not fully understood, there are likely multiple factors contributing to cognitive decline, including neurotoxicity treatments, inflammation, disruption of brain structural networks, and comorbidities. ,
- ■
Treatment and Prophylaxis: There are no known pharmacologic treatment or preventive options for chemo-brain. Case reports using modafinil and methylphenidate to improve cognitive function have been published with limited results, but randomized trials are needed to determine their place in treatment. ,
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree