Neuroendocrine tumors (NETs) represent a spectrum of histologies which share the ability to secrete hormonal or vasoactive peptides.1 The most common NETs are carcinoid and pancreatic NETs. At the time of diagnosis, liver metastases are common and a negative predictor of survival.2 Furthermore, liver metastases can cause debilitating symptoms due to both mass effect and secretion of vasoactive peptides. A subset of these patients present with the classic carcinoid syndrome which includes diarrhea, flushing, bronchospasm, and valvular heart disease.3
Management of neuroendocrine liver metastases is optimized with a multidisciplinary approach that includes medical oncology, surgery, and interventional radiology. This allows for various treatment options to be discussed by subspecialists and a comprehensive treatment plan employed. Image-guided therapies, namely thermal ablation and transarterial embolization, are used both as an adjunct to surgery and when surgery is not a viable option.4 These techniques have been shown to be effective for both local tumor control and symptom relief.5 This chapter reviews the various image-guided therapies currently available for NET liver metastases with regard to technique, efficacy, and available outcomes data. In addition, emerging and novel image-guided therapies are also reviewed.
Ablation of neuroendocrine liver metastases is used for similar indications as surgical resection, typically in the setting of low tumor burden when surgical resection is not an attractive option.6 The 2012 National Comprehensive Cancer Network (NCCN) guidelines include ablation in the management of both carcinoid and pancreatic neuroendocrine liver metastases.7 Although surgery remains the gold standard, thermal ablation can be used for deep-seated lesions not amenable to wedge resection, when a nonsurgical minimally invasive procedure is preferred, or in patients who are poor surgical candidates.8
During thermal ablation procedures, one or more probes are advanced into target tumors under image guidance (Fig. 130-1). Once appropriate probe positioning is confirmed, thermal necrosis is induced in an elliptical zone surrounding the probe tip. The most common thermal ablative techniques for NET liver metastases are radiofrequency ablation (RFA) and microwave ablation (MWA), which have largely replaced cryoablation and percutaneous ethanol injection.9,10
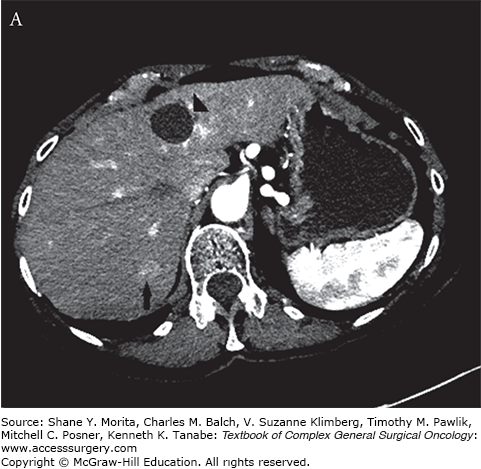
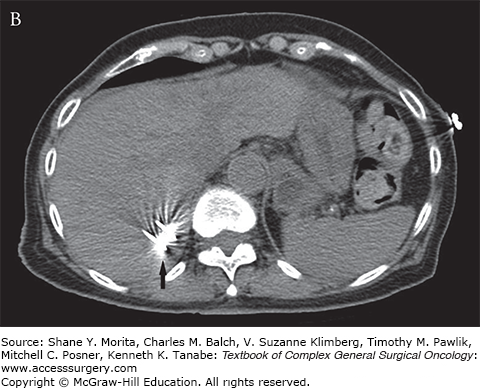
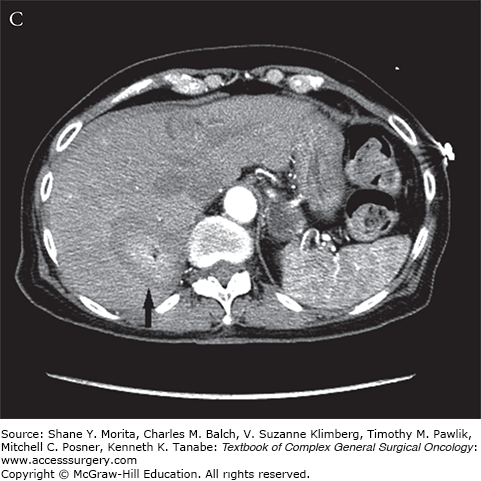
FIGURE 130-1
A. Arterial phase contrast-enhanced CT demonstrates an arterially enhancing neuroendocrine liver metastasis (arrow). A hepatic cyst (arrowhead) is incidentally noted. B. CT image demonstrating tips of two microwave probes (arrow) centered within the neuroendocrine metastasis. C. Contrast-enhanced CT demonstrating postablation changes at the target lesion (arrow). A wide margin was created as the lesion was only visible on arterial phase imaging.
Prior to the procedure date, a careful review of cross-sectional imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) is critical in order to identify all lesions and ensure ablation is technically feasible. Patients are typically seen in the outpatient clinic in order to explain the procedure and identify comorbidities or other factors such as anticoagulation therapy that may affect the procedure.
Percutaneous ablation can typically be performed on an outpatient basis. Routine preprocedural antibiotics have been recommended by some authors; however, there is currently no consensus on their use for liver ablation.11 With regard to coagulation parameters, the Society of Interventional Radiology Consensus Guidelines recommend an INR of <1.5 and platelets > 50,000/µL for liver ablation.12 Both aspirin and clopidogrel are recommended to be held for 5 days prior to the procedure. Octreotide therapy should be considered prior to ablation as preformed hormones can be released during treatment.
Once in the interventional suite, initial imaging is performed to identify the target tumors. The procedure can be performed under either general anesthesia or moderate sedation depending on clinical factors, patient comorbidities, and/or operator preference. CT is the most common modality used for percutaneous image guidance, though PET/CT, US, and MRI can be utilized as well.
Ablation is most successful for lesions measuring less than 4 cm in size.8 As lesion size increases, the incidence of local tumor recurrence also increases.13 Beyond the zone of thermal necrosis, a rim of incomplete necrosis forms up to 8 mm in depth.14 For this reason, an ablation margin of at least 1 cm is recommended. For larger lesions, multiple probes are typically used to achieve an adequate margin. In addition, probes are designed to create slightly different size and morphology of burn zones. This allows the operator to select a probe most appropriate for the target lesion.
Although some groups prefer RFA owing to longer experience with the modality, MWA offers several theoretic and practical advantages based on its different mechanism for tissue heating. RFA utilizes rapidly alternating electric currents to induce ionic frictional heating.15 Heating of the surrounding tissue depends on the propagation of electric current, which can be impeded as charred or desiccated tissue builds up around the probe. In addition, a heat-sink effect can occur near flowing vessels which carry heat away from the ablation zone, thereby reducing the effectiveness of the treatment.
Microwave ablation, however, utilizes electromagnetic radiation (900 to 2400 MHz) which directly heats tissue via dielectric hysteresis.16 The microwave radiation propagates through tissue regardless of electrical impedance and therefore can heat beyond charred tissue. This mechanism of heating is less prone to heat sink and has been shown to induce a larger zone of ablation in shorter time.17 Finally, with the use of multiple probes, MWA can treat multiple lesions simultaneously resulting in a shorter overall procedure. Multiple lesions must be treated sequentially with RFA.
Outcomes data is more robust for RFA compared to MWA since microwave is a newer technology. In 2010, a group from the Cleveland Clinic reported on 13-year experience of neuroendocrine liver metastases treated with RFA via a laparoscopic approach.18 A total of 547 lesions in 89 patients were treated during 119 sessions. The group chose RFA when metastatic disease involved less than 20% of liver volume and treated lesions up to 10 cm in size. Of the 39 patients with hormonal symptoms prior to ablation, 97% reported at least partial relief after ablation and 73% had complete relief. The response lasted a median of 14 ± 5 months (range, 1 to 106). The local recurrence rate per lesion was 7.4% and 24 patients (27%) underwent repeat RFA. The mean hospital stay was 1.1 days. Perioperative morbidity was 5.6% and there was one 30-day mortality secondary to intra-abdominal sepsis. Although the authors of that study prefer the laparoscopic approach, both surgical and percutaneous approaches are effective with similar reported outcomes.19 The surgical approach has the advantage of identifying lesions not evident on cross-sectional imaging, while the percutaneous approach is less invasive with shorter hospital stay and recovery time.20
In 2013, a large, multi-institutional report was published reviewing the outcomes of 473 MWAs (61 for NET metastases) targeting 875 hepatic lesions.21 The overall ablation success rate was 97% (839 of 865). For the neuroendocrine subgroup, median recurrence-free survival was 33.0 months and median overall survival was 91.9 months.
Overall, the percutaneous approach resulted in shorter median hospital stay when compared to laparoscopic and open surgical approach (1 vs. 2 vs. 5 days, p = 0.0001) without a difference in morbidity rates (11.1% vs. 9.3% vs. 15.9%, p = 0.292). Local recurrence rate was higher with percutaneous ablation compared to laparoscopic and open (14.6 vs. 6.0 vs. 4.9, p = 0.014), but this did not result in a difference between progression-free or overall survival. To our knowledge, there are currently no randomized studies comparing RFA vs. MWA for neuroendocrine liver metastases.
Complications of thermal ablation can occur secondary to probe placement or as a result of thermal injury. Probe placement can result in damage to adjacent bowel or vessels along the probe tract. Bleeding at the ablation zone is rare owing to the coagulative effects of thermal ablation. Some operators may choose to ablate the access tract during probe removal if there is concern for bleeding.
Thermal ablation itself can result in a reactive pleural effusion when the lesion is near the hepatic dome. With lesions near the hilum, special care must be taken to avoid thermal injury of bile ducts, which can result in stricture or fistula formation. The large blood vessels, however, are relatively protected by flowing blood. Following ablation, abscess formation within the ablation cavity can occur as necrotic tissue serves as a nidus for superinfection.
The complication rates are similarly low for both RFA and MWA. One recent report of 736 patients treated with MWA for both primary and metastatic liver malignancy found a major complication rate of 2.9% and minor complication rate of 7.3%.22 There were no procedure-related mortalities. The most common major complications were pleural effusion requiring drainage (n = 3), intraperitoneal hemorrhage (n = 2), and colonic perforation (n = 2). In 2004, Curley et al23 reviewed the complication rates of 608 patients with malignant liver tumors treated with RFA. The reported early complication rate was 7.1% and late complication rate 2.4%. The most common complications were symptomatic pleural effusion (n = 11), ablation cavity abscess (n = 5), and probe tract hemorrhage (n = 4). There were three procedure-related mortalities (0.5%).
Neuroendocrine liver metastases derive their blood supply from the hepatic arteries, while normal hepatocytes derive most of their blood supply from the portal vein. Transarterial therapies can thus target NET metastases with relative sparing of normal liver. Transarterial therapies play a central role in the management of NET liver metastases since the majority of patients are not amenable to surgical resection and/or ablation at the time of diagnosis.5 Furthermore, because NET liver metastases invariably recur after resection, there is often a role for transarterial therapies even after successful surgery and ablation.6,24 These therapies include transarterial embolization (TAE), transarterial chemoembolization (TACE), and selective intra-arterial radiation therapy (SIRT or radioembolization). Each of these therapies are discussed in detail in the following sections.
Similar to that discussed previously with thermal ablation, planning for TAE begins with clinical consultation and review of cross-sectional imaging. Clinical consultation is important for identifying comorbidities and for managing patient expectations. Routine laboratory and coagulation testing is performed. For transarterial procedures, a goal international normalized ratio (INR) of <1.5 and platelets >50,000 is recommended. Clopidogrel is held for 5 days prior to the procedure and low-molecular-weight heparin (LMWH) dose held on the day of procedure. Aspirin therapy may be continued depending on the clinical scenario.12
Transarterial therapies are typically performed under moderate sedation and with local anesthesia at the arteriotomy site. Broad-spectrum antibiotics are recommended prior to the procedure.11 In addition, octreotide therapy is typically initiated before and during embolotherapy to prevent potential adverse effects (i.e., malignant hypertension) of preformed hormones that may be released with tumor necrosis. The arterial puncture is made in the femoral artery distal to the inguinal ligament and overlying the femoral head. Puncture at the level of the femoral head is crucial to allow for adequate compression and hemostasis at the conclusion of the procedure.
Prior to embolization, angiography of the celiac and superior mesenteric arteries is performed to identify hepatic arterial anatomy. In one review of 600 patients, only 61% of patients had traditional hepatic arterial anatomy with left and right hepatic arteries originating solely from the proper hepatic artery.25 Successful embolization depends on treatment of all tumor-feeding vessels. In addition, extrahepatic arteries commonly arise from the hepatic arteries, particularly the left. In one study of 250 patients, extrahepatic arterial branches were found in 205 patients, most commonly the right gastric (n = 196), hepatic falciform (n = 129), and accessory left gastric (n = 43) arteries.26 Failure to identify extrahepatic arteries originating from the hepatic arteries can lead to nontarget embolization and morbidity.
Once target arteries are identified, a wire and catheter are advanced into the desired location where embolization is to be carried out. When bilobar disease is present, each lobe is typically treated with independent procedures to reduce the risk of liver failure.5 Patients with tumor burden greater than 75% of the liver volume are at greatest risk for liver failure.27 In these cases, embolization should be performed over several treatment sessions targeting only a small section of the liver at a time.
After embolization is concluded, the catheter is withdrawn and the arterial sheath is pulled. Hemostasis can be obtained with approximately 10 minutes of manual compression or with one of several commercially approved closure devices. Both TAE and TACE result in the postembolization syndrome of elevated liver enzymes, abdominal pain, nausea/vomiting, and fever. This typically resolves in 48 to 72 hours. For this reason, most patients are admitted to the hospital for at least one night following embolization for symptom management. Transarterial radioembolization typically causes milder symptoms and is more commonly performed on an outpatient basis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






