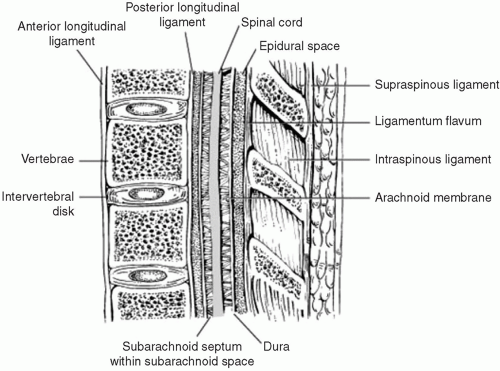
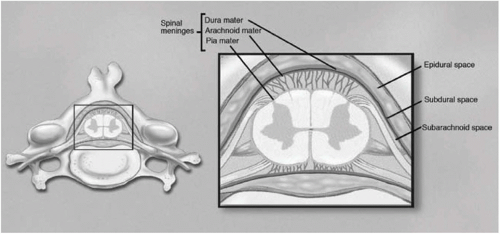
Intractable cancer pain has been routinely treated with the neuraxial (intrathecal or epidural) infusion of opioids for more than a quarter-century. Spinal and supraspinal sites are the areas at which intrathecal or epidural morphine injections are effective.
25 Initial reports indicated high efficacy and few complications or side effects.
26,
27,
28,
29,
30 At that time, many believed that the solution to intractable pain was in hand and widespread application awaited only the development of suitable long-term delivery systems.
31 Subsequently, additional case series and surveys made it clear that neuraxial administration of opioids was not risk free,
32 that tolerance to neuraxial opioids could be as big a problem as tolerance to oral or systemic opioids,
33,
34,
35 and that not all patients with pain from cancer experienced good pain relief with neuraxial opioids.
18,
36,
37 The failure rate of epidural and intrathecal opioids in cancer pain can be as high as 30%.
38,
39 Techniques of drug delivery also seemed to play a role in outcomes. Gourlay et al
40 reported that bolus dosing of neuraxial opioids resulted in less tolerance than a continuous infusion. On the other hand, Hassenbusch et al.
41 reported that chronic epidural morphine infusions were associated with very little tolerance development. As the field of neuraxial opioids developed, it became clearer that there were distinct differences between epidural and intrathecal administration, some related to drug pharmacokinetics, others to anatomy and technique advances and that the intrathecal route was better suited to treatment at home by continuous infusion than the epidural route.
42 Route of administration is now recognized as a critical factor for neuraxial opioid administration.
Epidural Administration
Although the objective of epidural administration is to affect pain relief by altering spinal cord dorsal horn synaptic transmission, there is some systemic absorption by all epidurally-administered drugs, including opioids.
43 One of the most important factors governing the spinal bioavailability of epidurally-administered opioids is their ability to redistribute out of the epidural space and into surrounding tissues. The kinetics of drug movements among epidural, intrathecal, and plasma compartments are complex, and the epidural and intrathecal spaces cannot be viewed simply as two compartments separated by a single barrier.
44 CSF and plasma pharmacokinetics of opioids do not parallel their epidural pharmacokinetics and the hydrophobic character of opioids governs multiple aspects of their lumbar epidural pharmacokinetics. A significantly larger proportion of the epidural dose of morphine reaches the CSF than is the case for any of the other more lipid-soluble opioids.
44The utility of each drug to be administered epidurally must be determined by studying whether the observed analgesia is due to selective action on the spinal dorsal horn or is related to systemic uptake of the drug and effects upon sites other than the spinal cord, such as the brain or peripheral nerves. Obviously, combinations of these two effects are likely for many opioids. Morphine is the standard drug against which all others are compared for efficacy and side effects. It was the first opioid administered into the epidural space that was active on the dorsal horn. Studies have shown conclusively that it produces analgesia by its actions on the dorsal horn of the spinal cord.
45 Subsequent studies have shown that highly lipophilic opioids, such as fentanyl, sufentanil, or alfentanil are inappropriate for epidural administration because of their rapid systemic uptake and effects outside of the spinal dorsal horn.
46,
47,
48,
49,
50Reports documenting the use of different opioids in the epidural space are now available; morphine is clearly the drug of choice for the majority of patients for this application. However, the long-term superiority to systemically administered drugs has been debated. In 1989, Sjogren reported no significant differences between oral and epidural routes of administration in long-term treatment of 14 cancer patients when pain relief, sedation, and reaction times were assessed.
51 Hassenbusch et al. studied the efficacy and safety of continuous epidural morphine administration in 69 patients with cancer pain in the midline abdomen/pelvis or lower extremities who did not respond adequately to systemic opioid treatment.
52 The detailed characteristics of the pain syndromes were not described for each patient. An epidural catheter was placed for evaluation of efficacy of morphine: 60% of this group reported good pain relief and had an internalized catheter and Infusaid pump (a pump with a fixed infusion rate) implanted. Before the administration of epidural drug, the patients reported a visual analog scale (VAS) of 8.6 ± 0.3. One month after implantation of the catheter and pump, the VAS scores were 3.8 ± 0.4 (
P < .001). Long-term benefits were described: 80% of the implanted patients reported satisfactory pain relief (at least a 30% reduction from preimplant VAS levels) at 3 and 6 month reevaluation. Also, the systemic opioid requirements were significantly decreased compared to preimplantation levels: by 79% at 1 month and 64% at 9 months. Some tolerance was seen: the mean epidural dose was 20.7 ± 2.6 mg/day 1 month after implantation and rose to 49.3 ± 9.9 mg/day 9 months after initiation of this treatment strategy. This study had a very low complication rate and reported no
instances of respiratory depression, epidural infection, meningitis, epidural scarring, or catheter failure. This was one of the seminal studies that demonstrated good longterm results with epidural morphine infusion in patients with pain from cancer.
By contrast, other studies have revealed a much higher failure rate with morphine.
38,
39 Nine years’ experience with epidural morphine in 146 cancer pain patients was reported by Samuelsson et al.
53 The mean treatment time for each patient was 92 days and epidural opioids were the sole pain treatment in 53% of patients. They reported that the mean dose of epidural morphine was 18 mg/day with a range of 6 to 120 mg/day. At the termination of this treatment strategy, the mean daily dose was 69 mg/day with a range of 2 to 540 mg/day. Absence of an initial satisfactory response to epidural morphine was noted in 25 patients. Withdrawal from initially successful therapy was observed in 27 patients; an additional nine patients experienced catheter failures and five reported drugrelated reasons for termination of epidural morphine. Those most likely not to respond to epidural morphine were patients with neuropathic pain states, those with movement-induced incident pain, those with visceral pain and those with painful cutaneous ulcerations.
Our review of the literature and our own experiences suggest that epidural opioid administration is not likely to be a successful long-term therapy for pain associated with cancer, particularly if it is the sole analgesic therapy. The major issue is the development of fibrosis around the epidural catheter that impedes drug delivery and results in local pain at the time of injection.
54 Tolerance does occur, but it is not usually an unmanageable problem. Maintenance of the catheter in a favorable position can also be a problem. We strongly prefer intrathecal administration of drugs in setting of cancer pain, as we believe it a more reliable method of drug delivery and it does not, in our hands, have increased risks.
Intrathecal Administration
Intrathecal drug delivery offers significant advantages for pain relief over epidural administration of opioids. These include: uniform drug distribution within the CSF (as long as highly lipophilic drugs are not used), superior efficacy of pain relief, relative absence of systemic effects at clinically useful intrathecal doses, ease and certainty of catheter insertion by CSF aspiration or contrast agent injection, and lower reported incidence of complications. As in the epidural space, the behavior of opioids in the intrathecal space is governed largely by their lipid solubility. Hydrophobic opioids have a very large apparent volume of distribution compared with that of more hydrophilic opioids.
55 Rapid elimination of hydrophobic opioids from the CSF results in very limited rostral spread in CSF. In addition, the bioavailability of these opioids at spinal cord sites more rostral than the site of administration is also very limited. This explains why delayed respiratory depression is observed clinically with morphine but not with fentanyl or sufentanil.
The ratio of intrathecal dosing to oral dosing is approximately 1 to 300, thereby significantly reducing the systemic opioid exposure. Implanted catheters and pumps can be used to infuse any opioid in aqueous solution, including morphine, hydromorphone, methadone, meperidine, fentanyl, and sufentanil. The intrathecal delivery route has been the subject of numerous reports with somewhat differing conclusions.
2,
15,
18,
36,
52,
56,
57,
58,
59,
60,
61,
62,
63,
64 Most of these reports describe significant pain relief from intrathecal opioids, but this literature suffers from many deficiencies and a complete picture of the use of this technology is often difficult to ascertain. Few studies have defined pain relief in a prospective fashion using standardized instruments. Some studies have described a few patients who manifested no pain relief to intrathecal opioids in spite of often astronomical increments in dosing.60,63,65-66 In most cases we do not know if this was a failure of the delivery system or a pain that did not respond to opioid treatment. The actual rate of patients with cancer pain not responsive to intrathecal opioids is not known, but it is certainly not zero. Nor do we know with any precision the efficacy of intrathecal opioids for the relief of cancer pain.
A useful study by Paice
60 contrasted the pain relief from intrathecal opioids in patients with cancer and nonmalignant diseases. The best results were seen in patients with cancer pain of somatic origin. However, when compared to non-cancer pain patients, the average initial dose of opioid was higher in the patients with cancer. Whereas the average dose used by cancer patients rapidly increased and then reached a plateau, non-cancer patients seemed to exhibit a more gradual but constant dose increase over long periods of use. This study contrasted with that of Yaksh and Onofrio
64 who studied the changes in morphine infusion dosing in 130 cancer pain patients and reported that the initial average dose of 4.8 ±0.4 mg/day increased to 21 ± 9 mg/day after 1 year of therapy. Plummer’s study in 1991
11 indicated wide variations in the dose requirements to adequately control cancer pain. They indicated that the type of pain also played a significant role in the response to intrathecal opioids, similar to what was reported above for epidural opioids. Long-term intrathecal morphine infusions were studied by Gestin et al.
67 in 50 patients with advanced cancer and pain. The average infusion lasted 142 (range: 7 to 584) days and the mean starting dose was 2.5 (range: 0.4 to 8.3) mg/day; this increased to a mean final dose of 9.2 (range: 1 to 94) mg/day. The average dose was 5.4 (range: 1 to 23) mg/day and all the patients were said to have satisfactory pain relief. One interesting report suggested that methadone given intrathecally produced pain relief inferior to that seen with morphine, even with doses ten times the equivalent of morphine.
68
Adverse Effects of Neuraxial Opioid Therapy
The adverse effects of systemic morphine are well described in the medical literature. Neuraxial administration of this drug results in a similar range of side effects, despite the more limited distribution of the drug in the body. Some of these side effects are temporary, normally lasting for the first several days after initiation of therapy and then resolving; others are more enduring.
69 In a retrospective study of 82 patients receiving long-term opioid therapy for noncancer-pain, Winkelmuller and Winkelmuller
70 reported the most frequent side effects as
constipation (50%), disturbance of micturition (42.7%), and nausea (36.6%). These occurred early in the course of therapy and responded to appropriate medication. Some patients experienced a loss of libido or amenorrhea for the first 6 to 8 months of therapy, but these side effects proved self-limiting to most patients and disappeared after 12 to 14 months of therapy. In most series, common problems associated with intrathecal opioid therapy have included nausea, vomiting, pruritus, urinary retention, and constipation. Less common adverse events have been respiratory depression and opioid-induced hyperalgesia. Less frequently recognized by clinicians are problems associated with endocrine abnormalities (in particular, sexual dysfunction) and edema.
Abs et al.
71 reported on the endocrine consequences of long-term intrathecal opioid use and noted that, in patients receiving intrathecal opioids, the majority of men and all women developed hypogonadotropic hypogonadism, about 15% developed central hypocorticism, and about 15% developed growth hormone (GH) deficiency. In this study of 73 patients (29 men and 44 women; mean age, 49.2 ± 11.7 year) receiving intrathecal opioids, the mean duration of opioid treatment was 26.6±16.3 months and the mean daily dose of morphine was 4.8 (3.2 mg). Decreased libido or impotency was present in 23 of 24 men receiving opioids. Serum testosterone level was less than 9 nmol/L in 25 of 29 men and was significantly lower than that in the control group (
P < .001). The free androgen index was below normal in 18 of 29 men and was significantly lower than that in the control group (
P < .001). The serum luteinizing hormone (LH) level was less than 2 U/L in 20 of 29 men and was significantly lower than that in the control group (
P < .001). Serum folliclestimulating hormone (FSH) was comparable in both groups. Decreased libido was present in 22 of 32 women receiving opioids. All 21 premenopausal women developed either amenorrhea or an irregular menstrual cycle, with ovulation in only one. Serum LH, estradiol, and progesterone levels were lower in the opioid group. In all 18 postmenopausal women, significantly decreased serum LH (
P < .001) and FSH (
P = .012) levels were found. The 24-hour-urinary free cortisol excretion was below 20 microg/day in 14 of 71 opioid patients and was significantly lower than that in the control group (
P = .003). The peak cortisol response to insulin-induced hypoglycemia was below 180 µg/L in 9 of 61 opioid patients and was significantly lower than that in the nonopioid group (
P = .002). The insulin-like growth factor I standard deviation (SD) score was below -2 SD in 12 of 73 opioid patients and was significantly lower than that in the control group (
P = .002). The peak growth GH response to hypoglycemia was below 3 µg/L in 9 of 62 subjects and was significantly lower than that in the control group (
P = .010). Thyroid function tests were normal. No other metabolic disturbances were noted, apart from significantly decreased high-density lipoprotein cholesterol levels (
P = .041) and elevated total/high density lipoprotein cholesterol ratio (
P = .008) in the opioid group compared to the control group. Supplementation with gonadal steroids improved sexual function in most patients.
Water retention and peripheral edema caused by intrathecal opioids can be problematic, resulting in limitations of physical activity and the production of lymphedema, ulcerations, and hyperpigmentation of the skin.
72 Cephalad migration of opioids within the CSF and interaction with opioid receptors in the posterior pituitary gland with the release of vasopressin may explain opioidinduced fluid retention.
69 We have had patients whose fluid retention was so profound that their intrathecal opioids had to be discontinued.
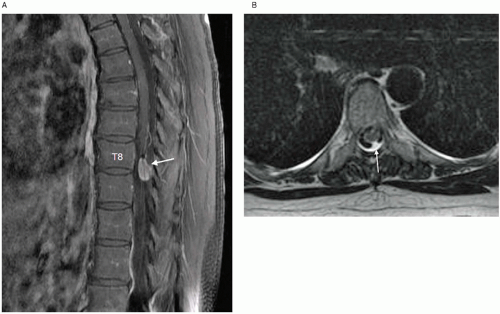
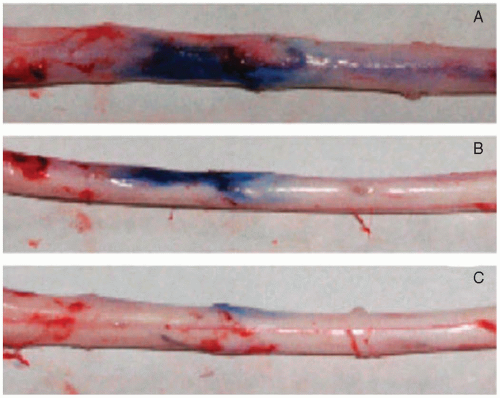
Catheter-tip inflammatory-like masses or granulomas have been attributed to implanted intrathecal catheters used for long-term pain management (
Fig. 22.3).
24 The first case was reported by North et al. in 1991.
73 Drug that precipitates from solution can cause an inflammatory mass and mimic a tumor in the subarachnoid space.
74 Preliminary experimental evidence suggests that dural mast cell activation and subsequent release of inflammatory mediators (such as cytokines and histamine) are responsible for catheter tip inflammatory mass lesions.
23,
75 Cases have occurred in patients receiving commercially prepared preservative-free morphine sulfate alone, as well as formulations of hydromorphone and morphine sulfate alone or in combinations with other analgesics and local anesthetics prepared by compounding pharmacies. The incidence of granuloma formation has been estimated to be 0.04% after 1 year of therapy, increasing to 1.15% after 6 years of therapy.
76 We suspect that the actual rate of granuloma formation is significantly higher; many cases have probably not been reported to central data banks and smaller granuloma may not be clinically apparent. High CSF drug levels of opioids, and in particular, drug concentration infused rather than absolute dose have been implicated as the cause of the inflammatory masses.
77 Yaksh et al.
78 noted 30% of granulomas developed in patients receiving less than 10 mg/day; 40% were using morphine concentrations of less than 25 mg/mL. Many of these patients were receiving drug mixtures, which may also be a key factor in granuloma formation.
Symptoms and signs consistent with inflammatory masses include diminished or loss of drug (analgesic) effect, new-onset radicular and back pain, and associated spinal cord neurologic deficits. Overt findings may include changes in the patient’s baseline neurologic condition, such as motor weakness (including gait difficulties), sensory loss (including proprioceptive loss), hyper- or hypoactive lower extremity reflexes, and any evidence of bowel or bladder sphincter dysfunction.
79 Imaging with a gadolinium-enhanced magnetic-resonance imaging (MRI) or computed tomography (CT) myelogram confirms the diagnosis. Plain MRI or CT scan or x-ray is of no value in establishing this diagnosis. Clinical evaluation based solely on the development of neurologic symptoms or decreasing analgesic efficacy alone in the absence of specific imaging studies lacks sufficient sensitivity to exclude the presence of catheter-associated masses.
80 Subtle prodromal symptoms and signs may include diminishing analgesic effects (loss of previously satisfactory pain relief) and remarkable or unusual increases in the patient’s underlying pain. Some patients may manifest frequent dose escalations in the attempt to recapture analgesic effects. In other cases, dose escalations and sizable drug boluses reduce the patient’s pain only temporarily or to a lesser degree than previous experience predicted. Gradual, insidious neurologic deterioration weeks or months after the
appearance of subjective symptoms was the most common clinical course before the onset of myelopathy or cauda equina syndrome.
24 Catheter tip granuloma formation is a potential neurologic disaster and must be considered as an emergent problem in any patient who manifests new symptoms and signs of pain or spinal cord dysfunction.
Escalating doses of intrathecal morphine may prove problematic because of increased risk for hyperalgesia and myoclonus.
57 Opioid-induced hyperalgesia is a pronociceptive phenomenon that can occur with acute or chronic opioid administration. This paradoxical effect is likely due to the opioid effects on a variety of cellular targets including N-methyl-D-aspartic acid (NMDA) receptors, spinal glutamate transporters, and protein kinase C81-82 and does not appear to be an opioid-receptor mediated event.
83 Ibuki et al.
84 demonstrated the increased content of excitatory amino acid neurotransmitter in spinal cord tissue with intrathecal morphine administration. Sakurada et al.
83 suggests that nociception associated with high-dose intrathecal morphine may mediated by morphine-3-glucuronide. Opioid-induced hyperalgesia is discussed extensively in
Chapter 19.
Bolus Versus Continuous Infusion of Opioids
The optimal method of delivery of neuraxial opioids (bolus vs. continuous infusion) is unclear. Controversies exist regarding the development of tolerance, differences in quality of pain relief, and duration of effect between the two modalities of administration. The behavior of opioids when administered as a slow continuous infusion is different from single injection bolus.
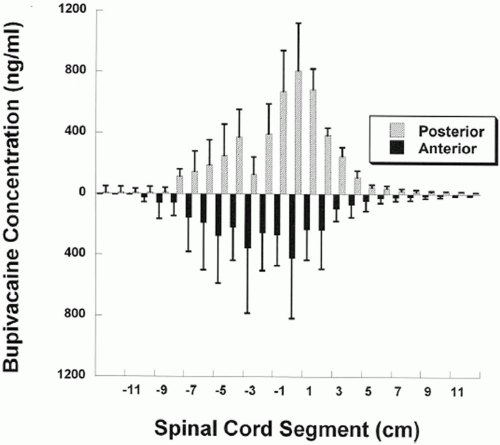
43 As a slow infusion, the concentration of morphine is limited to a short distance from the point of administration. This implies that the CSF is a very poorly mixed compartment. By contrast, a single bolus injection of intrathecal morphine has a prolonged and extensive neuraxial effect.
85Clinical effectiveness of intermittent bolus and continuous infusion of epidural opioids may not differ. In a study of 28 patients with severe cancer pain, these modalities were compared.
40 The mean duration of treatment was 169 (range: 6 to 537) days for continuous infusion patients and 140 (range: 28 to 378) days for repeated bolus doses. Both treatment modalities provided adequate pain control and the modalities were judged equally effective. Rauck et al.
86 evaluated the efficacy and safety of an intrathecal bolus delivery system of morphine in 119 cancer patients with cancer pain refractory to systemic opioids or uncontrollable opioid-related side effects. Pain relief, reduction in systemic opioid use, and reduction in opioid-related complications were evaluated together as a measure of overall success over a 16-month period. Patients were eligible if they had pain from cancer or cancer therapies or experienced pain that could only be controlled at doses that caused intolerable side effects. In addition, patients were enrolled if they had a life expectancy of 4 months or greater. Although not reported directly, it appears that there were only nine patients remaining in the study by month 16. The median starting bolus was 1 mg of morphine and the median average daily dose was 1.8 mg/day.
Median bolus size and median average daily dose increased steadily to 2.5 mg and 5.1 mg/day, respectively, at 4 months. Average numeric analog scale pain decreased from 6.1 to 4.2 at 1 month and was maintained through month 7 (
P < .01) and through month 13 (
P < .05). Systemic opioid use was significantly decreased throughout the study (
P < .01). The majority of patients in this present study decreased their overall opioid requirements by greater than 50% when using intrathecal opioids; many discontinued systemic use entirely. There was a pronounced decrease in the incidence of opioid-related side effects with intermittent intrathecal dosing compared to previous systemic dosing. Overall success (>50% reduction in numeric VAS pain score, use of systemic opioids, or opioid complication severity index) was reported in 83%, 90%, 85%, and 91% of patients at months 1, 2, 3, and 4, respectively. The most commonly recorded complications were sleep disorders, daytime drowsiness, constipation, and nausea. Frequency and severity of sleep disorder, daytime drowsiness, urinary retention, and constipation were significantly improved by 1 month, and five of the eight complications were significantly improved at 4 months.
Tolerance to the antinociceptive effect of morphine is well established in most mammals.
87 Tolerance occurs for a number of reasons, including previous opioid exposure, changes in pain modality or intensity, and psychological factors. However, in patients with cancer, it may be difficult to determine the precise reason for dose increase because of the often progressive nature of the disease and associated increase in pain. Systemic morphine via repeated subcutaneous injections can result in substantial tolerance within 3 days.
87 By contrast, tolerance develops more slowly to morphine at localized spinal sites.35,88-89 Animal studies with chronic intrathecal catheters and continuous infusion of morphine over a period of days demonstrate a dose-dependent antinociception that declines over the infusion interval implying the development of opioid tolerance.
90 Tolerance produced by morphine infusion is dependent on an increase in local phosphorylating activity by protein kinase C in the dorsal horn of the spinal cord.
91 Human studies of repeated intrathecal or epidural morphine administration have produced conflicting results. Ventafridda et al.
18 observed a rapid loss of effectiveness of 1 mg of morphine during a 3- to 5-day sequence of once-daily intrathecal injections in cancer patients. In a retrospective study of the doses of morphine administered intrathecally by chronic infusion in a population of 163 patients (130 patients had pain that was metastatic in origin) treated by 19 physicians, Yaksh and Onofrio
64 reported a time-dependent increase from 4.8 ± 0.4 mg/day at week 1 (n = 130) to 16 ± 4 mg at 24 weeks (n = 33) and to 21 ± 9 mg at 52 weeks (n = 10). However, 48% of patients infused for periods in excess of 3 months showed less than a twofold increase in dose by 3 months. In a series of 35 patients treated with continuous intrathecal morphine (mean = 5.4 months) for intractable cancer pain, Penn and Paice
61 reported that the occurrences of tolerance were infrequent and could be managed effectively. A retrospective review of 159 patients with refractory cancer pain treated with daily boluses of intrathecal morphine demonstrated only a moderate increase in intrathecal opioid dose that did not limit the patients’ ability to obtain adequate relief.
92 The mean follow-up period was 95 days (range: 5 to 909 days), the mean starting dose of intrathecal morphine was 2.69 mg (range: 1 to 7.5 mg), and the mean terminal dose was 7.82 mg (range: 1 to 80 mg).
In summary, these studies suggest that, although tolerance to the effect of spinal morphine may develop in the cancer patient, tumor progression is much more likely to increase opioid requirements in a patient with opioidresponsive pain.
Assessment of Long-Term Efficacy of Neuraxial Opioids
The long-term benefits of epidural and intrathecal opioid therapy for cancer pain have been difficult to objectively assess for many reasons:
The criteria for treatment success are often vague and frequently vary between reports. There have been no standardized outcomes criteria.
The patient selection criteria for initiation of neuraxial opioid therapy have varied widely. In some centers, neurologists or oncologists are primarily responsible for pain management. They may be more content with systemic analgesics and more conservative with invasive measures than pain management specialists. They may refer patients for consideration of neuraxial therapies late in the course of the illness, when metastases have already become widespread, nociceptive inputs are high, and tolerance can be a significant problem. Frequently, such patients require supplementary local anesthetics for pain control; opioids alone are inadequate. By contrast, other centers, where pain management specialists have primary responsibility of cancer pain management, may initiate spinal therapy at an early stage, possibly even when systemic therapy may be equally if not more effective.
Cancer patients frequently have more than one type or location of pain, and the outcomes data are not specifically related to an identified pain site or type.
Meaningful statistical inferences cannot be made from the data as presented.
Some patients who experience inadequate pain relief and/or intolerable side effects from systemic opioids can obtain pain relief without side effects with intrathecal or epidural administration of the same or different drugs. There will be additional patients who never experience adequate pain relief with neuraxial opioids (so called “treatment failures”—
vide infra). Different types of pain in patients with cancer may respond differentially to spinal opioids.
93 Arner and Arner
94 studied the differential effects of epidural morphine in patients with cancer pain. They utilized intermittent bolus doses of morphine in 55 patients with different patterns of cancer pain (daily dose range: 4 to 480 mg; dose interval range: 3 to 12 hours). Twenty-eight of the 55 patients (51%) became pain free. Another 21 patients were completely relieved of one of two pain types, whereas other coexisting pain types were either unaffected or only partially relieved. Six patients reported no pain relief. Continuous pain originating from deep somatic structures led to the best outcomes with epidural morphine. Continuous visceral pain or
intermittent somatic pain originating from such things as a pathologic fracture, led to less predictable outcomes. Pains that were cutaneous in origin, thought to be neuropathic, or secondary to intestinal obstruction rarely responded. Coexisting pains that were not related to the underlying malignancy were not alleviated at all in ten patients. This study and others
53 suggested that there was some predictability for pain relief based upon the character of the pain after treatment with epidural morphine.
Intracerebroventricular Opioids
It is possible to deliver opioids directly into the cerebral ventricles using an intracerebroventricular (ICV) catheter attached to a subcutaneous reservoir. A neurosurgeon is required for catheter placement. Morphine sulfate has been the most commonly administered drug; it has a dramatic increase in potency in the ICV route when compared to intrathecal or epidural infusions. The ICV route may affect supraspinal pathways for analgesia that are not accessible to spinally administered opioids.
99 ICV morphine doses have been reported from 50 to 700 µg/day.
100,
101 The most commonly utilized drug administration system consists of a battery-driven infusion pump, implanted subcutaneously in the anterior abdominal or chest wall, and connected by subcutaneous tubing to the ventricular catheter that delivers the drug into the lateral ventricle.
Another administration strategy has been to connect the ICV catheter to an Ommaya reservoir and utilize intermittent percutaneous drug injections. ICV injections appear to last significantly longer than intraspinally administered drug. Some patients have been reported to obtain excellent pain relief via an implanted ventricular catheter connected to a subcutaneous Ommaya reservoir with 1 to 2 injections per day.
15 ICV drug delivery is particularly useful in head and neck cancer pain. Its use has also been reported in patients who initially manifested a favorable response to intraspinal infusions of opioids and subsequently development tolerance and were nearing the end of life. ICV injections or infusions manifest similar safety and side effects to intraspinal infusions, with the exception that an increased risk of respiratory depression exists during the first 3 days of therapy.
15Some refractory head and neck cancer pains do not respond to spinal opioids but will respond to ICV opioids. By contrast, pains below the waist are more amenable to spinal drug administration. Ballantyne et al.
102 performed a meta-analysis of 1,587 cancer patients, comparing ICV to the more commonly performed epidural and intrathecal opioid administration. All patients included had intractable cancer pain that had not adequately responded to systemic treatment. This study showed that sedation and confusion was observed in 4% to 5% of patients receiving ICV therapy. Pruritus, urinary retention, and persistent nausea occurred more frequently with spinal administration than with ICV therapy. Patients receiving ICV morphine required an initial dose of 0.25 to 2.0 mg/day. Onset of pain relief occurred in 2 to 30 minutes, and the typical duration of pain relief after a single dose was 12 to 48 hours. Tolerance seemed to be less of a problem than with either epidural or intrathecal therapy. Dose escalation was very gradual (the average increase in daily dosage was 0.375 mg/month). In a subsequent study,
Ballantyne et al.
103 reported on 72 uncontrolled trials in cancer patients assessing ICV (13 trials, 337 patients), epidural catheters (31 trials, 1343 patients), and subarachnoid catheters (28 trials, 722 patients). These uncontrolled studies suggested excellent pain relief in 73% of ICV patients compared with 72% of those with epidural and 62% of those with subarachnoid catheters. Unsatisfactory pain relief was infrequent in all three treatment groups. Side effects, commonly persistent nausea, persistent and transient urinary retention, transient pruritus, and constipation occurred more frequently in patients with epidural and subarachnoid catheters. On the other hand, respiratory depression, sedation, and confusion were more common with ICV. The incidence of major infection when implanted pumps were used with epidural and subarachnoid catheters was zero. Reported complications with ICV therapy were less than with epidural or subarachnoid catheters. ICV administration of opioids should be considered in patients with head and neck cancer pain that does not respond adequately to intraspinal delivery of drug.