(1)
Department of Pathology Beckley Veterans Medical Center (West Virginia), McGuire Veterans Medical Center, Virginia Commonwealth University, Richmond, VA, USA
Review of Pertinent Histology and Physiology of Peripheral Nerve Sheath
Schwann cells play a very important role in the many aspects of the peripheral nervous system. Schwann cells have well-developed cellular processes which allow to wrap around axons and form tight junctions with each other [1–3]. Each axon is sheathed by one layer of Schwann cells which connect to each other at the node of Ranvier. This wrapping property might underlie the wavy appearance of nuclei seen in nerve sheath tumors. If one visions the Schwann cell arrangement in a nerve fascicle, the resemblance to the Verocay body characteristic of schwannomas becomes evident. Apparently, the neoplastic cells are polarized and supported by basal laminin on both sides.
The nerve sheath also contains mast cells which probably play a role in the angiogenesis and formation and maintenance of the blood–nerve barrier. The interaction between mast cells and Schwann cells is through the production of paracrine factors [4]. Through the production of TGF beta, mast cells influence the collagen production of nerve sheath cells. Mast cells are frequently seen in neurofibromas, less so in schwannomas.
Key Morphological Feature of Well-Differentiated Malignant Peripheral Nerve Sheath Tumors
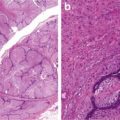
Hypercellular fascicles, sheets
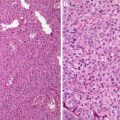
Diffuse nuclear enlargement, hyperchromasia (Fig. 4.1)
Fig. 4.1
Low-grade malignant peripheral nerve sheath tumor. Note cellular crowding, nuclear enlargement, and hyperchromasia
Discussion
Corresponding to decreased SOX10 and S100 expression, most malignant peripheral nerve sheath tumor cells show little schwannian or perineurial differentiation [3, 5]. Only at ultrastructural level, some schwannian differentiation manifests in some low-grade tumors. Instead, fibroblastic differentiation becomes evident. The tumor cells have underdeveloped cellular processes and cell junctions. Along with the absence of long spacing collagen and diminished basal laminin material, the cell process underdevelopment allows cells to unfold to form fascicular and sheet structures [2, 6–9].
The diffuse nuclear atypia manifests as nuclear enlargement and hyperchromasia. It should not be confused with the focal degenerative atypia which is common in benign nerve sheath tumors.
Differential Diagnosis
Neurofibroma
Typically, neurofibromas have the characteristic wavy nuclei and shredded carrot-type collagen which prevent the cells to form well-formed cellular fascicules or sheets. Focal atypia is degenerative in nature (bizarre nuclei with smudgy texture). In cellular neurofibromas, the cells tend to have more cytoplasm with or without well-formed fascicles. The plexiform variant might form fascicles. The fascicles however, are less cellular (more fibrous and mucinous).
Schwannoma
Schwannomas are usually easy to diagnose due to their characteristic Antonin A and B areas. They also are encapsulated and contain hyalinized blood vessels. Cellular schwannomas can form fascicles, contain focal necrosis, and even show significant bone erosion. The fascicles are however less cellular and are composed of bland cells. Additional features include encapsulation, subcapsular lymphocytic infiltration, as well as hyalinized vessels. The plexiform variant may also show fasciculation and even lack encapsulation. The fascicles are also hypocellular and composed of bland cells.
In difficult cases, a panel of immunostainings might help. Benign nerve sheath tumors are positive for p16 and p27 and show low reactivity for p53 and Ki6 [10, 11]. In general, they have a mitotic rate less than 4/10 HPF. Also, they show diffuse S100 reactivity in contrast to the weak and focal staining seen in malignant cells.
Review of Pertinent Histology and Physiology of Astrocyte
Astrocytes
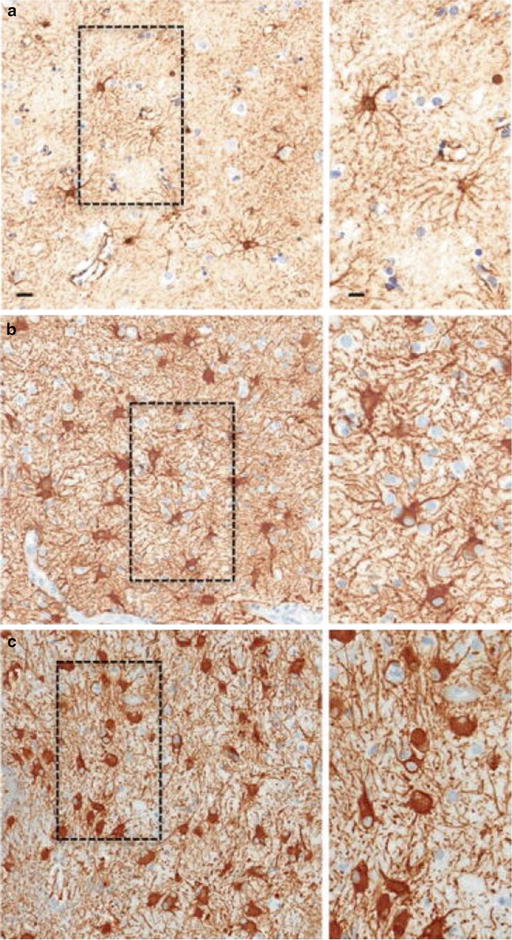
Astrocytes are the key player in the central nervous system. They are involved in virtually every aspect of the business of the system. Importantly, through their highly specialized cellular processes and cell junctions, they provide a highly sophisticated framework for the system allowing the formation of different functional domains, the blood–brain barrier [12–15]. Moreover, through the non-overlapping arrangement of the exuberant arborization of cellular processes, each cell has its distinctive cellular domain which can be brought out with a GFAP stain.
Not only are astrocytes an important component of the blood–brain barrier; they also control the angiogenic/vasculogenic process in the system [16–18]. Probably as a check and balance mechanism in order to prevent oxidative stress in this highly metabolically active system, glial cells also are equipped with means to rein in the angiogenic/vasculogenic process. This mechanism is likely to be powerful and preserved in gliomas. As circumstantial evidence, microvascular hyperplasia characteristic of grade IV gliomas represents ineffective angiogenesis probably as a result of compromise between high levels of counteracting factors. To circumvent it, glioma cells develop multiple mechanisms for neovascularization [16, 18].
Astrocytes are heterogeneous and the extended family members express common astrocytic markers but have morphological and functional differences to accommodate specific structural and physiological needs.
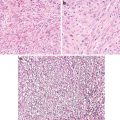
Key Morphological Features for Diffuse Gliomas (WHO Grade II)
Increased cellularity due to cell domain disruption
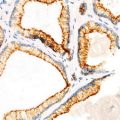
Infiltrative nature (Fig. 4.2)
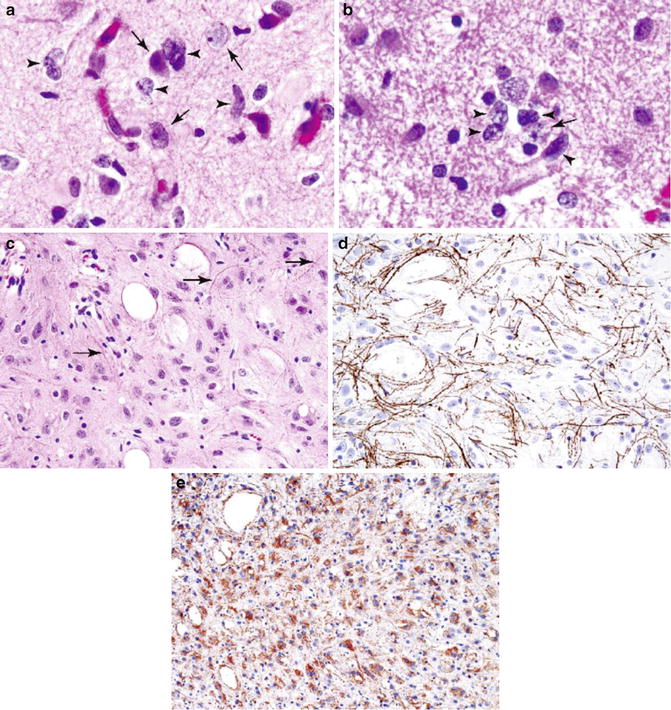
Fig. 4.2
Infiltrative astrocytoma, WHO grade II. Note infiltrative border with cortical neurons (a, b; arrows, cortical neurons; arrowheads, tumor cells). Microcystic changes (c). Neurofilament protein immunostaining highlights axons (d). Glial fibrillary acid protein stain lights up neoplastic astrocytes with poorly developed cellular processes (e) (Practical Surgical Neuropathology, Elsevier/Churchill Livingstone, 2010 with permission)
Discussion
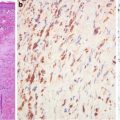
The infiltrative gliomas represent a spectrum of malignant glial cell tumors characterized by well-defined morphological features. Grade II tumors are characterized by moderately increased cellularity. Tumors of higher grades have increased mitotic activity and cytological anaplasia (grade III) and necrosis or microvascular hyperplasia (grade IV). Localized gliomas are a group of benign glial tumors which have distinct molecular, histological, and anatomical features, reflecting astrocyte heterogeneity.
Corresponding to decreasing expression of GFAP, glioma cells have decreased development of cellular processes and GAP junctions. Importantly there is a grade-dependent decrease in the expression of GAP junction protein connexin 43 [19–21]. This important protein plays an essential role in glial morphology, proliferation, and motility. Underdeveloped cellular processes and cell junctions allow cells to get close (hypercellularity in a fixed bony cavity) and migrate (infiltration of normal tissue). This is in contrast to the hypercellularity in reactive gliosis. In mild to moderate gliosis, the characteristic cellular domain is maintained by the non-overlapping arrangement of the exuberant process arborization [12, 22]. Only in severe diffuse gliosis the orderly cellular domain is disrupted probably through overexpression of GFAP and connexin 43 which bring cells closer to each other by allowing intermingling and overlapping of cellular processes (Fig. 4.3). Therefore, the contrasting morphological differences can be highlighted by a GFAP stain. With the stain, even the difference between reactive and neoplastic gemistocytic cells can be appreciated.