Needle Localization and Radioguided Occult Lesion Localization Techniques: Introduction
In parallel with the increasing use of mammographic screening and ultrasonography, nonpalpable breast lesions have been diagnosed with increasing frequency over the past 2 decades and at present constitute approximately 20% of all breast lesions.1
Nonpalpable lesions are a challenge to breast surgeons, because they must be precisely localized preoperatively and subsequently excised with clear margins and without excessive removal of breast tissue if the excision is merely diagnostic. Microcalcifications often found by screening mammography are frequently an early sign of neoplastic proliferation and must also be completely excised, an intervention that can forestall the development of infiltrating breast carcinoma.2 There are various methods for localizing these lesions: hooked wires, carbon particles, cutaneous markers, and large-molecular-weight colloids labeled with radioisotope.2 All are in current use. Needle localization is the most widely and frequently used method.3-5 The aim is to obtain accurate lesion localization and simultaneous surgical removal of the lesion with adequate margins.
A guide needle containing a stainless steel wire (Kopans wire) hooked at its distal end is inserted into the lesion under ultrasonic, mammographic, or magnetic resonance imaging (MRI) guidance. Ideally the guide needle should pass through the lesion and extend approximately 1 cm beyond. The wire must be long enough to protrude from the skin after insertion and withdrawal of the guide needle.8 Lesions revealed by ultrasound are best localized under ultrasonic control.9,10 Similarly, lesions detected only by MRI can now be localized using MRI guidance, and these procedures are becoming more refined.11 Once the correct position of the wire has been confirmed, the guide needle is carefully withdrawn. At this point, the hook in the wire reforms and anchors it in the tissue.8 More than 1 needle can be inserted into a single breast, for example to mark an extensive lesion by delimiting its borders. After localization, a 2-view mammogram (craniocaudal [CC] and mediolateral oblique [MLO]) is performed and sent to the operating room with the patient to document the location of the lesion and aid the surgeon with incision planning. The location of the target lesion within the breast is determined by estimating the distance from the nipple in both views; the CC view is used to determine whether the lesion lies medial or lateral to the nipple, and the MLO view is used to determine whether the lesion lies superior or inferior to the nipple. The depth of the lesion is also noted. Using the localization films in this manner allows the surgeon to estimate the location of the lesion in 3 dimensions and to place the surgical incision over the lesion itself, avoiding unnecessary dissection or tunneling through the breast if the entry site of the wire is in another quadrant of the breast.
Once proper incision placement is determined and the procedure is underway, the surgeon follows the wire down to the lesion site and removes the lesion together with the wire.8 If the incision has been placed away from the entry site of the wire, a superficial flap is raised at the level of the breast parenchyma to bring the wire into the field of the dissection, and the wire is then traced down to the site of the lesion. The removed specimen is marked with radiopaque clips or threads to define its orientation. Specimen radiography is performed if the needle localization procedure was performed for microcalcifications or if preoperative diagnosis was obtained with image-guided biopsy with clip placement. In the former case, the specimen x-ray serves to document that the targeted microcalcifications have been removed, and in the latter case, to document that the biopsy clip is contained within the specimen. Radiopaque clip marking of the specimen and placement onto a Plexiglas support, with the posterior margin placed accurately onto the Plexiglas surface, makes it possible to assess the distance of the lesion from the margins, and if clinically indicated, additional tissue may be excised. Needle localization can also be performed on breasts subjected to augmentation: the technique of Ecklund is used, and rates of lesion removal are close to 100%.12
The most common complications in order of occurrence are displacement of the wire from the breast, patient discomfort and pain, incorrect wire positioning, pneumothorax (rare), and wire migration to lung or abdomen (very rare).10
Another complication is that the wire occasionally breaks so that residual fragment of wire may be left within the breast.13-15
In 1996, the European Institute of Oncology, Milan, developed a new method for localizing occult lesions, called radioguided occult lesion localization (ROLL), the idea for which came from experience with radiocolloid injected close to the breast lesion (typically intradermally) to identify the sentinel node. The radioactive particles travel within the lymphatic ducts to be taken up by sentinel lymph nodes, usually in the axilla. It was noticed that some radioactivity always remained at the injection site, and the idea developed that, if the particles were too large to pass into the lymphatic system, all would remain at the injection site. Further, if this material were injected directly into a nonpalpable lesion, the radioactivity would remain there and serve as a beacon during surgical removal.
Studies showed that macroaggregate human albumin colloid, the particles of which have a larger average diameter than the microaggregate used in sentinel node biopsy, were practically immobile. After technetium 99m (99mTc) has been chemically bound to the particles, they could be injected directly into the occult lesion under stereotactic mammographic or ultrasonic control. In the operating room, a gamma ray–detecting probe locates the lesion and has proven invaluable in guiding its complete removal.16,17 After a pilot phase during which the quantity of radioactivity to be injected was optimized, ROLL became the method of choice for locating nonpalpable lesions at the European Institute of Oncology. It is effective, easily reproducible, and has a short learning curve.18
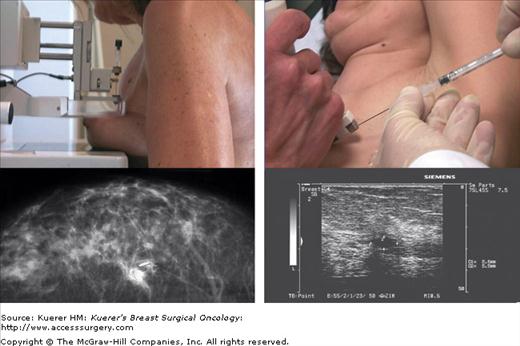
Twenty-four hours before the operation, human albumin macroaggregate (particle size 10-150 nm in diameter; Macrotec, Sorin Biomedica, Saluggia, Italy) labeled with 3.7 MBq of 99mTc in 0.2 mL of saline is injected directly into the lesion under ultrasonic or mammographic control (Fig. 55-1).
For lesions detected ultrasonically, the radiotracer is injected under the guidance of a linear probe attached to a needle biopsy device, which is inserted into the breast manually. The needle tip is positioned at the center of the lesion, as shown by a change of echogenicity at the lesion site. Radiotracer is then injected, followed by an additional minimal quantity of saline to flush the needle and help avoid dispersing the radioactivity. For lesions visible by ultrasound and mammography, injection under ultrasonic control is preferred.
For microcalcifications or other anomalies revealed only mammographically, mammographic equipment attached to a computerized stereotactic system is used to guide injection. Again a small quantity of pure saline (0.2 mL) is injected, and this is followed by a minimum quantity of radiopaque contrast medium via the same needle. The needle is then removed, and a standard orthogonal mammogram is taken a few minutes later to verify exact correspondence between the lesion and injected radiotracer/radiopaque material (Fig. 55-2). If a lesion has been completely removed by preoperative mammotome biopsy, the clip is located by mammography and the radiotracer injected at the clip site under stereotactic guidance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree