Risk of RINV is defined primarily by the anatomic area receiving treatment as well as the type of treatment.38 The most commonly employed category includes four levels of risk: high, moderate, low, and minimal.37,38 High-risk therapy consists of total-body irradiation. Moderate risk includes the upper abdomen, which carries a 60% to 90% risk of emesis without prophylaxis. Low risk includes the lower thoracic region and pelvis as well as the cranium (with radiosurgery) and craniospinal therapy. Minimal risk includes cranium, head and neck, extremities, and breast.
Highest Therapeutic Index
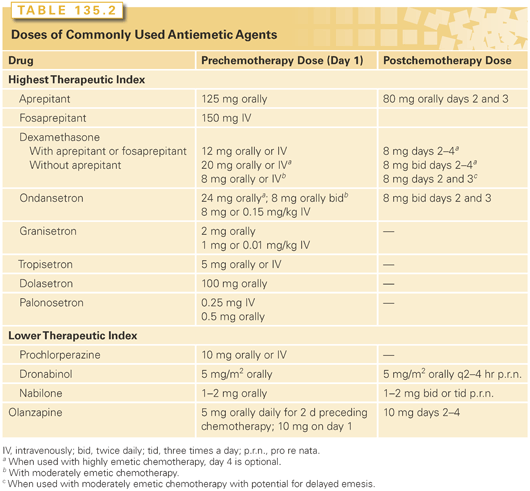
There are several classes of drugs that are effective in the prevention of CINV (Table 135.2). Those of highest therapeutic index include the 5-HT3 receptor antagonists, the NK1 receptor antagonists, and corticosteroids. The most widely available first generation 5-HT3 receptor antagonists include dolasetron, granisetron, ondansetron, and tropisetron. They have been shown to be superior to high-dose metoclopramide40 and have replaced it as first-line therapy in prevention of acute emesis. They have proven to be most effective in the prevention of acute CINV in the settings of highly and moderately emetogenic chemotherapy. All first-generation 5-HT3 receptor antagonists are considered therapeutically equivalent and are used interchangeably.37,38,41 Oral formulations of the first-generation 5-HT3 receptor antagonists have similar efficacy to intravenous formulations.42
Palonosetron is a second-generation 5-HT3 antagonist that differs from other agents in this class by virtue of a longer half-life of 40 hours and a higher binding affinity to the 5-HT3 receptor. It has been compared with first-generation 5-HT3 antagonists in two randomized phase 3 trials in patients receiving moderately emetogenic chemotherapy.43,44 Both trials were designed as noninferiority trials and met their primary end point. Post hoc analyses indicated superiority for palonosetron in a number of parameters to the comparator agents. In one trial, palonosetron 0.25 mg was superior to dolasetron 100 mg in complete response (no emesis or use of rescue medications) during the delayed (24 to 120 hours) and overall study periods.43 In the other trial, palonosetron 0.25 mg was superior to ondansetron 32 mg in complete response during the first 24 hours, delayed period, and overall 5-day study period.44 A third phase 3 trial in patients receiving highly emetogenic chemotherapy also reached its end point of noninferiority in comparing palonosetron with ondansetron 32 mg.45 Unlike the moderately emetogenic trials, there was no significant difference between palonosetron and ondansetron in the control of acute or delayed emesis. Approximately two-thirds of patients also received corticosteroids at the discretion of the investigator in the highly emetogenic trial. A post hoc secondary subgroup analysis of the patients receiving dexamethasone demonstrated superior control of acute and delayed emesis in the group receiving palonosetron. In another phase 3 trial,46 patients receiving either cisplatin or the combination of doxorubicin or epirubicin and cyclophosphamide were randomized in a double blind fashion to either a single dose of palonosetron at a dose of 0.75 mg or a single dose of granisetron (40 μg/kg) on day 1 of chemotherapy. In this trial, steroids for acute and delayed emesis were mandated. Palonosetron was more efficacious than granisetron, with 56.8% of patients in the palonosetron group experiencing an overall complete response (no vomiting or use of rescue medications) compared with 44.5% in the granisetron group (p <0.0001). This was also true for delayed emesis (56.8% versus 44.5%, p <0.0001). Palonosetron was found to be comparable to granisetron in the acute phase, with 75.3% complete response in the palonosetron group versus 73.3% in the granisetron group (difference of 2.29, 95% confidence interval = 2.70 to 7.27; p not done). The results of the phase 3 trials with palonosetron suggest that this agent is superior in efficacy to the first-generation 5-HT3 antagonists when used as a single agent or with dexamethasone. The relative efficacy of palonosetron compared with first-generation 5-HT3 receptor antagonists when used with NK1 antagonists remains unknown given the lack of randomized data. Most of the studies done with palonosetron have used the 0.25 mg dose given intravenously. Boccia et al.47 compared three oral doses of palonosetron to the standard 0.25 mg intravenous dose in 651 patients receiving moderately emetogenic chemotherapy. Patients were also randomized to dexamethasone or placebo. The oral dose of 0.5 mg was found to be noninferior to the intravenous 0.25 mg dose in the acute and overall phase. However, none of the oral doses attained noninferiority to the intravenous dosing in the delayed emesis phase.
Corticosteroids are a mainstay in the prevention of both acute and delayed CINV. In a large meta-analysis that included data from >5,000 patients, dexamethasone, the most commonly employed corticosteroid, was found to significantly improve the prevention of CINV compared with placebo or other antiemetics.48 Most of the studies involved the use of highly emetogenic chemotherapy. In the acute phase, it was estimated to increase the chance of complete prevention of vomiting by 25% to 30% versus placebo. Similar results were seen with delayed emesis. With moderate to highly emetogenic chemotherapy regimens, corticosteroids are typically not used alone but in combination with other antiemetics. Single-agent use is appropriate with mildly emetogenic regimens. The appropriate doses of dexamethasone for use with highly and moderately emetogenic therapy (in the absence of a NK1 antagonist) are 20 mg and 8 mg, respectively, based on the results of two large phase 3 trials.49,50
The most recent advance in CINV prevention has been the introduction of selective antagonists of Substance P’s binding to the NK1 receptor. Aprepitant is the first approved agent in this new class of antiemetics. In a phase 3 randomized study that included >500 patients, aprepitant significantly increased the overall 5-day complete response (no emesis or use of rescue) rate (72.7% versus 52.3%) when added to the standard therapy of ondansetron and dexamethasone in patients receiving high-dose cisplatin chemotherapy.51 Superiority for the aprepitant arm was demonstrated in both the control of acute and delayed emesis. Similar results were found in an identically designed phase 3 trial enrolling patients from Latin America.52 The addition of aprepitant may also improve the durability of antiemetic control over multiple cycles of cisplatin-based chemotherapy.53 Benefit for the addition of aprepitant has also been demonstrated in patients receiving a combination of an anthracycline and cyclophosphamide, a combination now recognized to be highly emetogenic. In a phase 3 trial in patients with breast cancer receiving an anthracycline plus cyclophosphamide, patients were randomized to an aprepitant-containing regimen, which included dexamethasone and ondansetron, or a standard regimen that consisted of dexamethasone and ondansetron.54 Aprepitant alone was used as delayed emesis prevention in the aprepitant arm, while ondansetron alone was used for delayed emesis protection in the comparison arm. A superior rate of complete response (50.8% versus 42.5%) during the 5-day study period was noted in the aprepitant arm.
A possible benefit for aprepitant in the prevention of CINV associated with moderately emetogenic chemotherapy has been demonstrated. Rapoport et al.55 reported the results of a phase 3 trial evaluating aprepitant with a variety of moderately emetogenic chemotherapy regimens as well as doxorubicin and cyclophosphamide. Patients were treated with aprepitant or placebo plus ondansetron and dexamethasone on day 1 and either aprepitant (experimental arm) or ondansetron (control arm) for delayed emesis. Overall, patients randomized to the aprepitant containing arm had significantly higher proportions of patients with complete response, including overall (62.8% versus 47.1%, p <0.01), in the acute setting (84.3% versus 72.5%, p <0.01), and in the delayed setting (64.8% versus 52.9%, p <0.01). Retrospective analysis was done comparing patients receiving doxorubicin/cyclophosphamide with those receiving moderately emetogenic combinations, which confirmed the superiority of aprepitant in those patients treated with doxorubicin/cyclophosphamide. For the non–doxorubicin/cyclophosphamide arm, while the addition of aprepitant statistically improved the proportion of patients who did not experience vomiting, the overall complete response rate did not differ between the aprepitant-containing group and the non–aprepitant-containing group. An additional trial prospectively evaluating aprepitant in moderately emetogenic chemotherapy did not demonstrate value for the addition of the NK1 antagonist. Hesketh et al.56 investigated the use of a single dose of casopitant, an NK1 receptor antagonist, in oxaliplatin-based chemotherapy. All patients were treated with ondansetron starting prior to oxaliplatin administration and continuing until day 3, dexamethasone day 1, and randomized to receive casopitant or placebo day 1. The primary end point was complete response (no vomiting and no use of rescue medications), and a total of 710 patients were included in the intention to treat analysis. Casopitant did not improve outcome compared to the dexamethasone and ondansetron regimen with similar rates of complete response overall (85 % in the placebo arm versus 86% in the casopitant arm; odds ratio = 1.1054; 95% confidence interval = 0.73 to 1.68; p = 0.6373), in the acute phase (96% in the placebo arm versus 97% in the casopitant arm), and in the delayed phase (85% in the placebo arm versus 86% in the casopitant arm). Given the limited and somewhat conflicting data on the value of aprepitant in the moderately emetogenic setting, the most recent European Society of Clinical Oncology-Multinational Association of Supportive Care in Cancer38 and American Society of Clinical Oncology (ASCO) antiemetic guidelines37 do not recommend the use of aprepitant with moderately emetogenic chemotherapy.
In 2008, an intravenous formulation of aprepitant (fosaprepitant) received regulatory approval for use with moderately and highly emetogenic chemotherapy. A single intravenous dose of fosaprepitant 150 mg was compared with 3-day oral dosing of aprepitant in a randomized double blind active-control designed trial and was found to be equivalent in efficacy in patients treated with cisplatin at doses of at least 70 mg/m2.57 This held true for both the delayed emesis period and the overall study period.
Of note, aprepitant is an inhibitor of the cytochrome P450 enzyme CYP3A4, which is involved in the metabolism of many drugs, including dexamethasone and certain chemotherapeutic agents. A pharmacokinetic study demonstrated that the plasma concentration of dexamethasone as measured as the area under the curve was more than two-fold higher with aprepitant compared with the same dose of dexamethasone without concomitant aprepitant.58 A dose reduction of dexamethasone from 20 to 12 mg (day 1) and 8 to 4 mg (days 2 and 3) yielded similar plasma dexamethasone levels to those in patients not taking aprepitant. The slowed metabolism of dexamethasone in the presence of aprepitant accounts for the recommendation to reduce dexamethasone doses by approximately 50% when administered with aprepitant.37 However, if the corticosteroids are given as part of the chemotherapeutic regimen rather than for antiemetic prophylaxis, the corticosteroid dose should not be adjusted.37 There is also a theoretical concern that chemotherapeutic agents that are metabolized by CYP3A4 may have a slowed clearance, leading to higher drug exposure, and increased toxicity. However, analysis of the completed phase 3 trials with aprepitant has not demonstrated a significant increase in adverse events in patients receiving aprepitant.
Recently, data on a new selective NK1 receptor antagonist netupitant has been presented. In a phase 2 study,59 694 patients receiving cisplatin-based chemotherapy were randomized to four study arms comparing three oral doses of netupitant (100 mg, 200 mg, 300 mg), all combined with oral palonosetron 0.50 mg, all given on day 1. All patients received oral dexamethasone on days 1 to 4. An exploratory aprepitant + ondansetron/dexamethasone arm was included. The study arm, combining 300 mg of netupitant with palonosetron, was superior to the palonosetron and dexamethasone arm in control of acute and delayed nausea and emesis as well as complete protection (p ≤0.05). A subsequent phase 3 study including 1,455 patients receiving cyclophosphamide combined with doxorubicin or epirubicin evaluated a fixed-dose tablet containing netupitant 300 mg combined with palonosetron 0.5 mg termed NEPA. Patients were randomized to receive NEPA or oral palonosetron 0.5 mg on day 1 prior to chemotherapy. All patients also received dexamethasone (12 mg NEPA arm; 20 mg palonosetron arm) on day 1. NEPA plus dexamethasone was found to be superior to palonosetron plus dexamethasone in rates of complete response during acute (p = 0.001), delayed (p = 0.047), and overall (p = 0.001) phases as well as in rates of no significant nausea.60
Lower Therapeutic Index
Drugs of lower therapeutic index include metoclopramide, butyrophenones, phenothiazines, cannabinoids, and olanzapine (see Table 135.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








