Fig. 2
For the nanoprecipitation method, precursor solutions are mixed to allow particle nucleation and growth. The resulting nanoparticles are insoluble in the solvent system whereas the individual precursors remain soluble; and therefore, the nanoparticles can be precipitated. This method was used to synthesize NMOFs composed of the anticancer prodrug c,c,t-Pt(NH3)2Cl2(succinate)2 (DSCP) and Tb3+ ions [48]. In this synthesis, the pH value of an aqueous solution of TbCl3 and [NMeH3]2DSCP was adjusted to 5.5, and methanol was added to the precursor solution to precipitate the nanoparticles. NMOFs formed by nanoprecipitation adopted spherical morphology with a diameter below 100 nm and carried an exceptionally high cisplatin loading compared to other nanocarriers. This method was used to synthesize a Zr-based NMOF containing DSCP by acetone-induced precipitation of a solution of ZrCl4 and DSCP in N,N-dimethylforamide (DMF) [49].
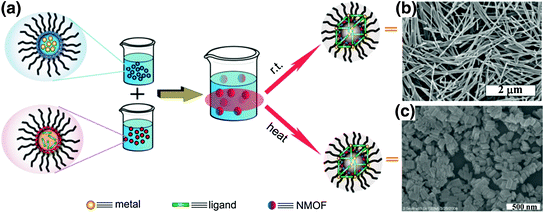
Solvothermal synthesis of NMOFs can be carried out by either conventional heating or using a microwave. Temperatures and heating rates are important parameters that control the NMOF nucleation and growth. An Fe3+ NMOF with the formula of Fe3(μ3-O)Cl(H2O)(BDC)3 was synthesized by heating a solution of FeCl3 and terephthalic acid (BDC) with a microwave [31]. The resultant crystalline NMOF displayed octahedral morphology with a diameter of 200 nm and adopted the known MIL-101 structure. A solvothermal reaction between pamidronate (Pam) and CaCl2 in diethylformamide (DEF)/H2O afforded single crystals of Ca-Pam with rod-like morphology of ~80 × 80 × 1000 nm in dimensions [50]. Similarly, microwave heating of a solution of zoledronate (Zol) and CaCl2 in DMF/H2O led to crystalline particles of Ca-Zol that adopts a rod-like morphology of ~70 × 70 × 1000 nm in dimensions [50]. The solvothermal method was also used to synthesize a UiO NMOF with the formula of Zr6(μ3-O)4(μ3-OH)4(amino-TPDC)6. This NMOF with amino-triphenyldicarboxylate (amino-TPDC) bridging ligand was synthesized by heating a DMF solution of ZrCl4 and amino-TPDC at 80 °C for 5 days, and exhibited a hexagonal-plate morphology with a diameter of ~100 nm and a thickness of ~30 nm [51].
Reverse microemulsions provide another method to control nucleation and growth kinetics of NMOFs because the building blocks for NMOFs are typically water-soluble. This surfactant-assisted synthesis can be carried out at room temperature or with heating. A crystalline Gd(BDC)1.5(H2O)2 nanorod was synthesized with this method by mixing two separate microemulsions containing either GdCl3 or [NMeH3]2[BDC] [52]. The particle morphologies could be controlled by adjusting the water to surfactant molar ratio (w value) of the microemulsion. Nanorods of 100–1000 nm in length and 35–100 nm in diameter were synthesized in microemulsions with different w values.
Surfactant molecules can be used to template the NMOF synthesis under solvothermal conditions by coating the surfaces of growing NMOF particles. For example, a reverse microemulsion of GdCl3 and [NMeH3]6[BHC] (BHC = benzene hyxacarboxylic acid) was heated at 120 °C to afford Gd-BHC NMOFs, which exhibited a blocklike morphology with dimensions of 25 × 50 × 100 nm and matched a previously reported lanthanide-BHC phase [40, 53]. This method was also used to synthesize NMOFs capped with lipid, such as 1,2-dioleoyl-sn–glycero-3-phosphate (DOPA). Several DOPA-coated NMOFs were synthesized by utilizing the surfactant-templated solvothermal reactions, including Zr-methotrexate (MTX) NMOFs, Zn-MTX NMOFs, La-DSCP NMOFs, and Zn bisphosphonate NCP containing platinum-based prodrugs [49, 54, 55].
The four general methods described above have been adopted to synthesize a large number of NMOFs. By independently adjusting NMOF precursors, reaction solvents, pH values, temperatures, surfactant, w values of microemulsions, a wide variety of NMOFs with well-defined morphologies and compositions can be readily synthesized.
2.2 Strategies to Incorporate Imaging or Therapeutic Agents Within Nanoscale Metal-Organic Frameworks
By taking advantage of the porous NMOF structure and flexibility of bridging ligands, several different methods have been developed to incorporate imaging or therapeutic agents within NMOFs with high loadings. These loading methods fall into two main categories: Direct incorporation during NMOF synthesis and postsynthetic loading [26, 27].
Imaging or therapeutic agents can be directly incorporated into the NMOF structure during synthesis as metal-connecting points/clusters or as the bridging ligand [33, 48, 52, 53, 56, 57]. Paramagnetic metal ions such as Gd3+, Fe3+, and Mn2+ serve as metal-connecting points in NMOF structures and can act as efficient magnetic resonance imaging (MRI) contrast enhancement agents. Platinum-based prodrugs (including DSCP, cis,cis,trans-[Pt(NH3)2Cl2(OCONHP(O)(OH)2)2], and Pt(DACH)Cl2[OCONHPO(OH)2]2), gemcitabine monophosphate, 2,3,5,6-tetraiodo-1,4-benzenedicarboxylic acid (I4-BDC) were adopted as bridging ligands to form NMOFs to provide chemotherapeutics cisplatin, oxaliplatin, and gemcitabine for cancer therapy and high Z element iodine for CT imaging [48, 55, 56]. This strategy can lead to NMOFs possessing very high agent loadings with uniform distribution throughout the frameworks. For example, NCP-1 and NCP-2 containing bridging ligands of cisplatin or oxaliplatin prodrugs achieved as high as 48 wt% cisplatin loading and 45 wt% oxaliplatin loading, respectively (Fig. 3) [55].
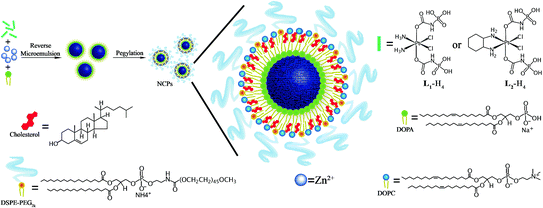

Fig. 3
General procedure of self-assembly of Zn bisphosphonate NCPs containing platinum-based prodrugs with lipid and PEG coatings [55]
The pores and channels in the NMOF structure allow postsynthetic loading of imaging or therapeutic agents. Biomedically relevant agents can be incorporated into the tunable pores of NMOFs by noncovalent or covalent interactions after the NMOF synthesis. A cisplatin prodrug, Pt(NH3)2Cl2(OH)(OEt), was loaded into UiO NMOFs via noncovalent encapsulation. Release studies showed sustained cisplatin release from the framework with negligible burst effects [51]. Férey and coworkers extended this strategy to NMOFs loaded with hydrophilic, amphiphilic, and hydrophobic agents [33]. Compared to noncovalent incorporation, postsynthesis covalent attachment of an agent offers a more robust approach in terms of preventing premature release of the agent. The agent covalently attached to the bridging ligand will only be released upon the decomposition of the NMOF structure. For example, Fe3+ NMOFs possessing 2-aminoterephallic acid (NH2-BDC) as bridging ligands allowed for the covalent attachment of either an optical contrast agent or a chemotherapeutic [31, 58].
2.3 NMOFs for Cancer Imaging
NMOFs have been evaluated for applications in magnetic resonance imaging (MRI), computed tomography (CT), and optical imaging [38, 59]. NMOFs show interesting and distinct advantages over other nanocarriers in delivering imaging contrast agents.
MRI is a noninvasive imaging technique based on the detection of nuclear spin reorientations in a magnetic field. MRI provides excellent spatial resolution, high soft tissue contrast, and unlimited penetration depth, but is intrinsically insensitive [32]. Therefore, large doses of contrast enhancement agents such as Gd(III) chelates are used in clinical scans to enhance the contrast between normal and diseased tissues [60]. The effectiveness of Gd(III)-containing NMOFs as T 1 -weighted contrast agents was first demonstrated by Lin and coworkers [52, 53, 61]. For example, Gd2(BDC)3(H2O)4 nanorods gave an r1 of 35.8 mM−1 s−1 in an aqueous xanthan gum suspension, which is almost an order of magnitude higher than that obtained with the commercially available T 1 -weighted contrast agent Omniscan [52]. Similar relaxivities were obtained for Gd2(BHC)(H2O)6 NMOFs synthesized using a surfactant-mediated method. Many of the Gd(III)-based NMOFs can also act as efficient T 2 -weighted contrast agents [53]. The relaxivity values of the NMOFs are dependent on nanoparticle size, with smaller NMOFs exhibiting larger r1 relaxivities, which can be attributed to the fact that smaller particles possess higher surface to volume ratios to enhance the exchange between Gd(III) bound water and bulk water. Boyes and coworkers further tuned the relaxivities of Gd(III)-based NMOFs by surface modification with polymers, showing that hydrophilic polymers led to an increased r1 relaxivity while hydrophobic polymers increased the r2 relaxivity [62, 63]. Unfortunately, Gd-NMOFs leached significant amounts of Gd(III) ions in water, which limited their in vivo applications as MRI contrast agents, due to the toxicity of free Gd(III) ions [64]. To address the toxicity issue, the Lin group and others have synthesized Mn(II)-based NMOFs and evaluated their applications as MRI contrast agents [65, 66]. Mn(II) ions have been shown to be potent MRI contrast agents with lower toxicity compared to free Gd(III). Nanorods of Mn(BDC)(H2O)2 were found to have an r1 and r2 of 5.5 and 50.0 mM−1 s−1 on a per Mn(II) basis at 3 T, respectively, whereas nanorods of Mn2(BTC)3(H2O)6 exhibited an r1 and r2 of 7.8 and 70.8 mM−1 s−1 per Mn(II) basis, respectively. Mn2(BTC)3(H2O)6 NMOFs were further coated with a thin silica shell and functionalized with a cancer targeting peptide to afford NMOFs with active targeting capability. This NMOF was evaluated in vivo and demonstrated an enhancement in T 1 -weighted signals in the liver, spleen, and aorta 1 h postinjection, which was attributed to the Mn(II) ions released from the NMOF. Horcajada and coworkers synthesized a series of iron-carboxylate NMOFs as T 2 -weighted contrast agents with sizes ranging from 50 to 350 nm and crystalline structure matching the MIL series. Negative enhancement of the liver and spleen was observed after injection of these NMOFs to Wistar rats, which dissipated 3 months postinjection, suggesting the accumulation, degradation, and subsequent clearance of the NMOFs [33].
CT imaging is based on attenuation of X-rays by a specimen to provide 3D images with high spatial resolution [67]. High Z number elements such as iodine, barium, and bismuth are chosen as CT contrast agents; however, large doses (tens of grams) are typically required in the clinic to achieve adequate contrast [67]. Lin and coworkers developed NMOFs constructed from Cu(II) or Zn(II) and I4-BDC as CT imaging contrast agents [56]. CT phantom studies showed that both NMOFs possessed slightly higher X-ray attenuation factors than commercially used contrast agent iodixanol. In another example, instead of incorporating the high Z element into the bridging ligand of the structure, Lin and coworkers incorporated high Z elements (Hf and Zr) into the M6(μ3-O4)(μ3-OH)4(RCO2)12 (M = Zr or Hf) secondary building units of UiO-66 NMOFs with high Hf (57 wt%) and Zr (37 wt%) contents [68]. Hf-NMOFs of different sizes were coated with silica and poly(ethylene glycol) (PEG) to enhance biocompatibility, and were used for in vivo CT imaging of mice, showing increased attenuation in the liver and spleen (Fig. 4).
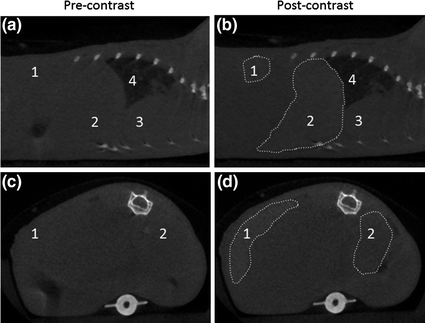

Fig. 4
Sagittal (a and b) and axial (c and d) CT slices of a mouse precontrast and 15 min after injection of Hf-NMOFs coated with silica and PEG. The areas of increased attenuation are outlined, and the labels are: 1-spleen (+131 HU), 2-liver (+86 HU), 3-heart, and 4-lungs [68]. Reproduced by permission of The Royal Society of Chemistry
A number of luminescent NMOFs have been synthesized, but their unfavorable absorption properties and low quantum yields have precluded the applications in biomedical imaging [38]. Lin and coworkers synthesized NMOFs containing a phosphorescent Ru(bpy)32+ derivative as the bridging ligand and Zn(II) or Zr(IV) as metal-connecting points [69]. Zr-based NMOFs with exceptionally high dye loadings (up to 57.4 wt%) were further coated with a layer of amorphous silica, functionalized with PEG, and an active targeting moiety for enhanced uptake in cancer cells. Confocal microscopy studies demonstrated an increased uptake of NMOFs in human lung cancer cells. Besides using optical imaging agents as bridging ligands for constructing NMOFs, optical dyes can also be incorporated within the frameworks post-synthetically. Kimizuka and coworkers created a series of NMOFs based on lanthanide ions and nucleotides [70–73]. Anionic dyes, such as perylene-3,4,9,10-tertacarboxylate, were incorporated within NMOFs of adenosine 5′-monophosphate and Gd3+. Confocal microscopy showed the uptake of this NMOF into the lysosomes of HeLa cells and its biodistribution in a murine model was examined. Lanthanide-nucleotide NMOFs were also used to successfully encapsulate other anionic dyes and negatively charged quantum dots. Recently, Lin and coworkers incorporated fluorescein isothiocyanate (FITC) into UiO NMOFs by forming thiourea linkage between isothiocyanate group on FITC and amino group of bridging ligand to afford FITC conjugated UiO NMOFs (F-UiO) with exceptionally high FITC loadings, efficient fluorescence, and excellent ratiometric pH-sensing properties (Fig. 5) [74]. Real-time imaging studies in live cells revealed endocytosis and exocytosis of F-UiO and endosome acidification in human lung cancer cells, which represents the first use of NMOFs for intracellular pH sensing and elucidating NMOF-cell interactions [74].
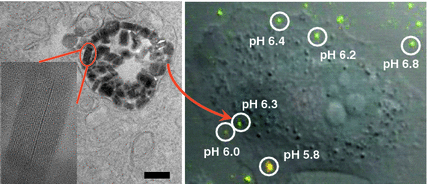

Fig. 5
High-resolution TEM image showing the distribution and structural integrity of UiO NMOFs in the endosomes of H460 cells. Inset is a zoomed-in view showing one intact UiO NMOF inside endosome. CLSM image was obtained from live cell imaging video, showing the overlay of green fluorescence (488 nm channel), red fluorescence (435 nm channel), and DIC. The pH of different endosomes or particles outside the cells was calculated based on the pH calibration curve obtained on live cells [74]. Reprinted with permission from [74]. Copyright (2014) American Chemical Society
2.4 NMOFs for Cancer Therapy
Our understanding of cancer biology has progressed enormously in the past two decades, bringing a large number of anticancer therapeutics, including small molecule inhibitors, antibodies, chemotherapeutics, and nucleic acid drugs, to the clinic [3, 75–77]. However, current therapeutics are limited by their nonspecific distribution throughout the body, leading to high doses, rapid clearance, poor pharmacokinetics, and undesired side effects [3, 4, 78]. NMOFs are potential nanovectors for delivering therapeutic agents to targeted areas of the body to overcome these limitations. Nanoscale dimensions of NMOFs allow them to take advantage of the EPR effect to achieve specific and enhanced accumulation in the tumor site. NMOFs can also control drug release with their large surface areas, high porosity, and presence of functional groups to interact with loaded moieties.
Lin and coworkers have developed a series of NMOF platforms to deliver platinum-based chemotherapeutics. An amorphous NMOF was built from a cisplatin prodrug DSCP and Tb(III) ions by the nanoprecipitation method, and further coated with a thin layer of silica and a silyl-derived peptide to actively target cancer cells [48]. This NMOF decomposes in physiological media and thus releases the cisplatin prodrug by diffusing out of the silica shell in a controlled manner to induce cytotoxicity in human colon cancer cells. The Iron(III)-carboxylate NMOF was post-synthetically modified to carry a cisplatin prodrug, c,c,t-Pt(NH3)2Cl2(succinate)(OEt), coated with silica, and targeted with RGD peptide targeting ligand [31]. Cytotoxicity was also observed for this NMOF. NMOFs constructed from a cisplatin prodrug DSCP and Zr(IV) or La(III) using surfactant-templated heating technique was capped with DOPA and modified with PEG [49]. These NMOFs showed cytotoxicity in human lung cancer cells. Recently, Lin and coworkers reported the self-assembly of zinc bisphosphonate NCPs carrying cisplatin and oxaliplatin prodrug with high drug loadings [55]. After PEGylation, these two NCPs showed minimal uptake by the mononuclear phagocyte system and excellent blood circulation half-lives of 16.4 and 12.0 h for NCPs carrying cisplatin and oxaliplatin, respectively. In multiple tumor xenograft murine models including colon, lung, and pancreatic cancer, NCPs exhibited superior potency and efficacy at very low drug dose compared with free drugs (Fig. 6).
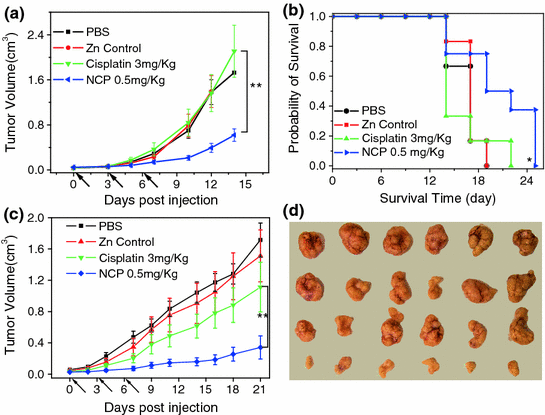

Fig. 6
In vivo antitumor activity of NCP carrying cisplatin. a In vivo tumor growth curves for NCP, free cisplatin, and other controls on the s.c. C26 tumor model. b Kaplan-Meier plots showing the percentage of animals remaining in the study with s.c. C26 tumor model. c In vivo tumor growth inhibition curves for NCP, free cisplatin, and other controls on the s.c. H460 tumor model. d Photos of resected H460 tumors from various groups. The last row is NCP group [55]
NMOFs can be loaded with other chemotherapeutic agents including methotrexate (MTX), pamidronate (Pam), and zoledronate (Zol), and showed cytotoxicity in multiple human cancer cells in vitro [50, 54]. Horcajada and coworkers encapsulated busulfan within the iron-carboyxlate NMOFs and demonstrated their comparable cytotoxicity as the free drug against several cancer cell lines [33]. Maspoch and coworkers encapsulated several anticancer drugs as guest species within NMOFs synthesized from Zn2+ and 1,4-bis(imidazole-1-ylmethyl)benzene [79]. Huang and coworkers synthesized coordination polymer sphere by combining 1,1′-(1,4-butanediyl)bis(imidazole) (bbi) and ferrous ions and conjugated folic acid to the surface [80]. The authors demonstrated the cytotoxicity of this coordination polymer sphere loaded with doxorubicin in HeLa cells.
MOFs have also been studied as carriers for gaseous molecules for anticancer therapy such as nitric oxide (NO). NO has shown anticancer activity in high concentrations and other activities including antibacterial, antithrombotic, and wound-healing applications. Morris and coworkers synthesized two MOFs from either cobalt or nickel and 2,5-dihydroxyterephthalic acid, which can absorb seven times the amount of NO than any previously reported material on a per gram basis via ligation to coordinatively unsaturated metal centers, with little background release [81].
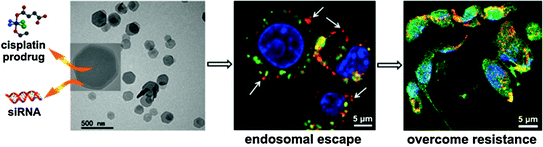
Most recently, NMOFs have been utilized for the delivery of nucleic acids. Lin and coworkers for the first time demonstrated the capability of a UiO NMOF to co-deliver cisplatin and small interfering RNA (siRNA) to drug-resistant ovarian cancer cells for overcoming drug resistance and enhanced anticancer efficacy [51]. A cisplatin prodrug was encapsulated into the channels of UiO NMOFs and siRNAs were attached to NMOF surface via multiple coordination bonds between phosphate residues on the siRNA backbone and vacant Zr sites on the NMOF surface. Co-delivery of cisplatin and siRNA with NMOFs led to an order of magnitude enhancement in chemotherapeutic efficacy in vitro (Fig. 7). Mirkin and coworkers synthesized UiO-66-N3 (Zr6O4OH4(C8H3O4-N3)6) NMOFs and covalently functionalized their surface with oligonucleotides utilizing a strain promoted click reaction between DNA appended with dibenzylcyclooctyne and azide-functionalized UiO-66-N3 [82]. When dispersed in aqueous solution, they exhibit increased stability and enhanced cellular uptake.
Several ongoing studies in the Lin group showed the potential of exploiting NMOFs as a versatile platform for combination therapies including chemotherapy, photodynamic therapy, and gene therapy. With more in-depth investigations, NMOFs are on the path through preclinical evaluations and hold great promise in anticancer therapy.
3 PSQ Nanoparticles
PSQ nanoparticles are formed via condensation of silanol-based monomers, yielding high drug loadings compared with other silica-based nanoparticles. PSQs have tunable structures and surface chemistry. PSQ particles can be considered as a special class of NMOFs/NCPs with Si as the metal-connecting point. Although not many examples are available for their synthesis and applications in the cancer imaging and therapy yet, the distinct properties of PSQ nanoparticles make them an interesting nanocarrier platform with significant potential in the clinic.
3.1 Synthesis of PSQ Nanoparticles
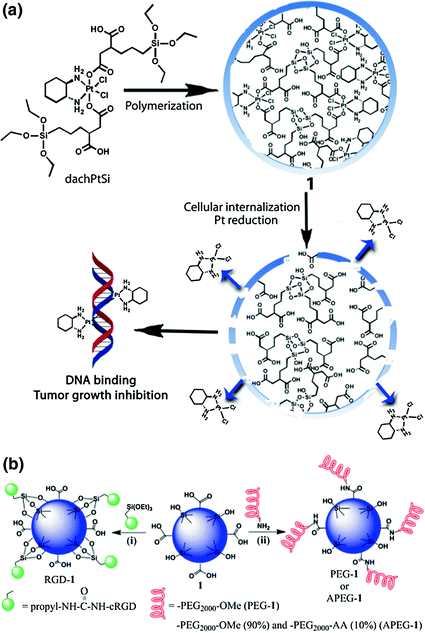
PSQs are hybrid materials composed of siloxane networks with organic or metal-organic bridging ligands. They are synthesized from bis(trialkoxysilanes) ((R’O)3-Si-R-Si-(OR’)3) via sol-gel reactions. As a homopolymer of (R’O)3-Si-R-Si-(OR’)3, PSQs allow much higher drug loadings than other silica-based materials and their physicochemical properties can be easily tuned by changing the monomer properties than in a silica-based materials. Lin and coworkers constructed PSQs carrying a platinum complex, [Pt(dach)Cl2(triethoxysilylpropyl succinate)2], further surface-modified the PSQs with PEG and anisamide ligand for active targeting to cancer cells (Fig. 8) [34].
3.2 PSQ Nanoparticles for Cancer Imaging
The cancer imaging applications of PSQs have been demonstrated by the Lin group [35]. Gd(III) chelates were covalently linked to PSQ particles via a labile disulfide bond, and the Gd-PSQ nanoparticles were post-synthetically coated with PEG and anisamide ligand to enhance biocompatibility and cell uptake in cancer cells (Fig. 9). The effectiveness of this PSQ nanoparticle as efficient optical imaging and MRI contrast agents were demonstrated in human lung and pancreatic cancer cells (Fig. 9).