Fig. 1
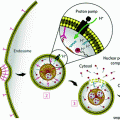
a–d Nanodiamond surfaces possess versatile chemical–physical properties that can mediate potent drug binding and marked improvements in MRI contrast efficiency levels. Reprinted with permission from Nature Publishing Group
Furthermore, the versatile chemical properties on the detonation ND surfaces enable either the conjugation or reversible adsorption of a broad collection of therapeutic and imaging compounds, depending on the intended application or indication being addressed [22–25]. Given these interesting properties and findings, NDs have emerged as promising platforms for drug delivery and imaging, particularly for applications in cancer, because they have improved the efficacy and safety of treatment and diagnosis of different types of cancer over conventional clinical standards via ND-drug synthesis methods that are reliable and scalable. These complexes were capable of mediating order of magnitude improvements in per-gadolinium (Per-GD(III)) relaxivity which are among the highest ever reported values compared to all clinical and nanoparticle agents [26]. In addition, ND-doxorubicin (NDX) agents are able to bypass drug-resistant breast and liver tumors to demonstrate marked improvements in tumor reduction capabilities with no apparent myelosuppression compared to the administration of doxorubicin (Dox) alone, which is a clinical standard [27].
While NDs come in different shapes and sizes, and both HPHT and detonation NDs have demonstrated significant promise in the basic and translational development domains, this chapter will focus on detonation NDs given that they have been extensively studied in preclinical models and proposed as platforms for magnetic resonance imaging (MRI). Current achievements as well as challenges toward clinical implementation will be addressed.
2 Nanodiamond Drug Delivery
Among the early demonstrations of the promise of NDs toward applications in biology and medicine was their use as chemotherapeutic delivery vehicles in vitro with Dox as the payload as well as other compounds [28–33]. Early studies demonstrated that while the Dox molecules were bound to the ND surfaces, their activity was attenuated such that the cytotoxic nature of Dox could potentially be reduced while adsorbed to the surface. The interactions between the ND surface and various classes of therapeutic compounds have been studied using various modeling/simulation methods to provide further insight into the unique chemical–physical properties of the ND facets [34, 35]. This was important in that the systemic toxicity of Dox can lead to significant side effects including myelosuppression, cardiotoxicity, superinfections, as well as mortality.
Therefore, this initial study paved the way for additional work that resulted in the preclinical validation of NDX safety and efficacy in liver and breast tumor models, preclinical anthracycline targeting toward TNBC, and localized glioblastoma therapy. Collectively, these findings have addressed particularly hard-to-treat cancers, demonstrating the promise of NDs as broadly applicable drug delivery platforms.
2.1 Nanodiamond Particles as Systemic Chemotherapeutic Delivery Agents for Drug-Resistant Breast and Liver Tumor Treatment
Drug resistance is a pervasive problem in cancer therapy that results in a vast majority of tumor treatment failure in metastatic cancer cases. Of all of the ND-drug agents that have been synthesized, ND-anthracycline agents are highly scalable and they potently bind the drug compounds that result in marked improvements in efficacy and safety. The ability to rapidly synthesize nanoparticle-tagged drugs that are too large to be effluxed from a tumor mass, coupled with an ability to prevent early systemic release and systemic toxicity and off-target effects would represent a very promising nanomedicine-based route to overcome drug resistance. A recent study demonstrated that NDX is among the most promising platforms for this indication due to the remarkably scalable nature of its synthesis protocol. NDX particles were systemically administered in mice via tail vein injection [27]. Subsequent toxicity measurements of serum IL-6 and serum ALT even at high ND doses indicated no apparent toxicity, demonstrating that the NDs were well tolerated. The impact of NDX injections on myelosuppression was evaluated. NDX administration resulted in no apparent myelosuppression, while the administration of Dox alone resulted in marked reductions in white blood cell count below threshold values. Myelosuppression is the dose-limiting side effect of chemotherapy and can cause major side effects such as superinfections and patient mortality.
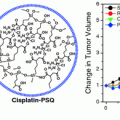
With regard to efficacy, unmodified Dox administration at 100 μg equivalence resulted in virtually no efficacy. NDX administration at the same dosage equivalence resulted in a marked decrease in tumor size. When the drug dosage equivalence was doubled, unmodified Dox administration resulted in accelerated animal mortality. When the lethal dosage was delivered via ND, all of the animals survived, and the tumor treatment efficacy was further enhanced, resulting in the smallest tumors observed in the study (Fig. 2). This demonstrated that the ND platform was capable of mediating major improvements to chemotherapeutic tolerance [27].
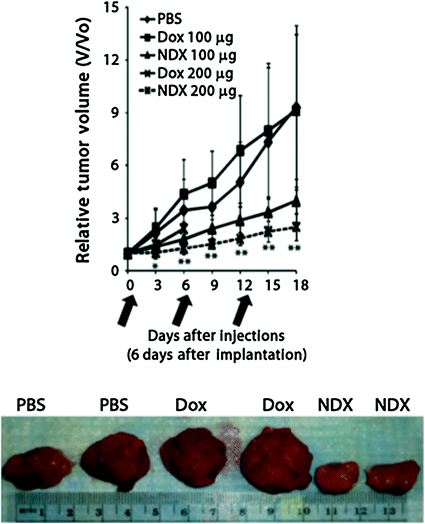

Fig. 2
Top NDX administration at 100 μg equivalence delays tumor growth compared to Dox alone. NDX at a doubled equivalent dose results in even further improved efficacy and drug tolerance. Bottom Unmodified Dox administration results in little to no efficacy given the drug resistant nature of the breast cancer studied. NDX administration results in markedly reduced tumor sizes. Reprinted with permission from the American Association for the Advancement of Science
This initial demonstration of preclinical NDX administration demonstrated that there was no premature drug elution even with a therapeutic that is bound via physisorption. This was confirmed by the fact that premature drug release would have resulted in both apparent myelosuppression due to the circulation of free drug, as well as no therapeutic efficacy since the Dox would be separate from the ND and effluxed. The absence of myelosuppression and marked improvements in efficacy confirmed that potent interaction of NDs with Dox served as an important mediator of both significantly enhanced efficacy and safety, even in a passively targeted system.
2.2 Liposome-Encapsulated Nanodiamonds for Targeted Triple-Negative Breast Cancer Treatment
The initial validation of NDX systemic administration as an effective strategy for drug-resistant breast and liver tumor therapy served as a promising passively targeted approach. The improved drug tolerance and therapeutic efficacy observed even with the lethal Dox dosage served as a foundation for the continued development of NDX toward clinical use. A recent study has synthesized a variation of the NDX complex in that the Dox compound was replaced with Epi [36]. The disease model in this study was the MDA-MB-231 triple-negative breast cancer (TNBC) that has been shown to overexpress the epidermal growth factor receptor (EGFR) [36]. Therefore, the ND-Epi complexes were encapsulated within liposomes that were surface functionalized with the EGFR antibody (Fig. 3). This architecture was employed in order to maximize drug loading on the ND surface due to the fact that the NDX study was able to confirm that the ND-anthracycline interaction was capable of enhancing intratumoral drug retention to prevent efflux and improve efficacy, circulatory half-life by a factor of 10, and eliminate myelosuppression, among other benefits. These ND-Lipid hybrid particles (NDLPs) thus served as an actively targeted variation of the ND-anthracycline complexes that were previously scalably synthesized [36].
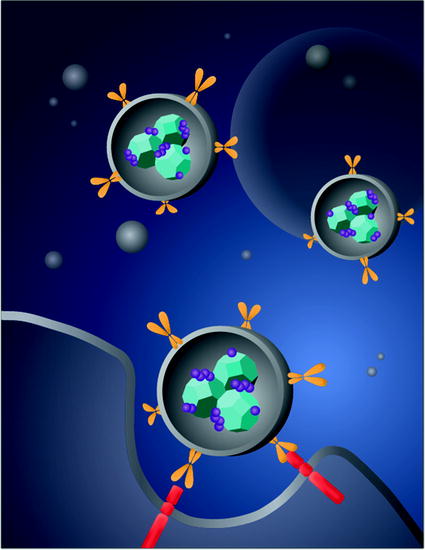

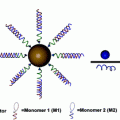
Fig. 3
A schematic demonstrates the synthesis of liposome-encapsulated nanodiamonds that are functionalized with epirubicin. The conjugation of antiepidermal growth factor receptor antibodies on the particle-enabled TNBC targeting. Image courtesy of Laura Moore
NDLP synthesis was accomplished using a rehydration process of lipid thin films that were comprised of cholesterol and biotinylated lipids and ND solution [36]. By harnessing biotin–streptavidin chemistry, the anti-EGFR antibodies were conjugated to the liposome surface. Characterization of NDLP synthesis was done using a broad range of methodologies including zeta potential analysis and dynamic light scattering (DLS) in order to assess particle size, Fourier Transform Infrared Spectroscopy (FTIR), flow cytometry in order to quantify successful NDLP synthesis and to differentiate the NDLPs from free NDs and lipids, and cryogenic transmission electron microscopy (cryo-TEM) in order to image NDLP formation. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) and enzyme-linked immunosorbent assay (ELISA) were used to assess lipid presence and confirm anti-EGFR antibody conjugation, respectively [36].
Comprehensive in vitro assessment of anti-EGFR functionalized NDLP targeting efficacy was performed. Epidermal Growth Factor (EGF) flooding studies resulted in gradually decreasing targeting efficacy as a result of increasing the EGF concentration which was a demonstration of successful anti-EGFR antibody conjugation and targeting abilities. With regard to preclinical efficacy, targeted (anti-EGFR antibody functionalized) and untargeted NDLP complexes, as well as phosphate buffered saline (PBS) and unmodified Epi were utilized as test conditions [36]. Unmodified Epi administration resulted in accelerated animal mortality that was similarly observed with unmodified Dox administration. Untargeted NDLP administration immediately improved drug tolerance as early mortality was no longer observed. Furthermore, tumor growth was clearly delayed. Interestingly, the administration of the anti-EGFR functionalized NDLPs resulted in tumor regression to the point where they were no longer visible, which is a promising finding for actively targeted ND-chemotherapeutic compounds (Fig. 4).
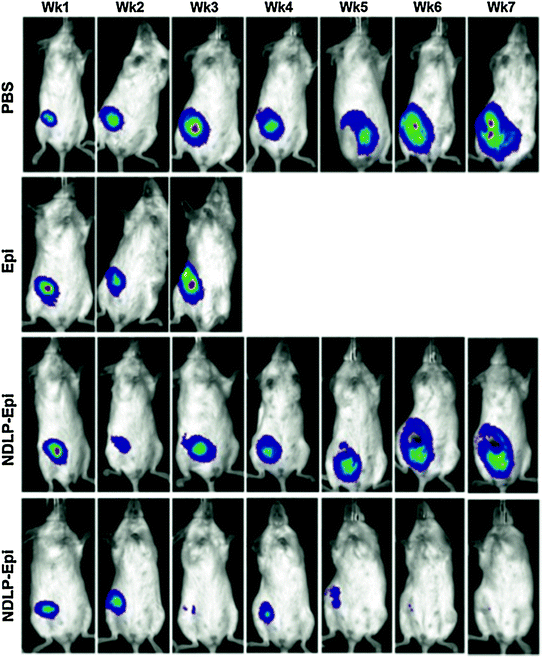

Fig. 4
Liposome-encapsulated nanodiamond complexes (NDLP-Epi) mediated tumor regression, while the administration of drug alone resulted in accelerated animal mortality. This study demonstrated that NDLP-Epi complexes were capable of improving drug tolerance in addition to efficacy. Reprinted with permission from Wiley-VCH
With regard to ND biocompatibility, this study provided among the most comprehensive assessments of ND serum and urine toxicity. A broad range of markers was analyzed including serum ALT, ALK and AST, hematocrit, blood urea nitrogen (BUN), bilirubin, white blood cell differential, hemoglobin, and red blood cell counts, among other readouts. In all cases, ND and NDLP administration resulted in no apparent toxicity that further substantiated the promise of the ND platform for drug delivery.
2.3 Nanodiamond-Based Glioblastoma Therapy
A key barrier to glioblastoma therapy is the need for the therapeutic to traverse the blood–brain barrier (BBB) [37]. In addition, off-target toxicity is of a major concern because nonspecific cell death in the central nervous system (CNS) results in irreversible damage since CNS neurons do not regenerate. Therefore, localizing drug release to the specific tumor-containing regions, when relevant, is of particular importance.
While the ability for NDX to cross the BBB should be further investigated, a recent study that utilized convection-enhanced delivery (CED), the stereotactic application of a catheter to directly deliver NDX into brain tumors demonstrated improved tumor localization and clear improvements to treatment efficacy in multiple tumor models [38]. Specifically, the C6 rodent model and U251MG, an aggressive human model, were both investigated in a rat model. Initial in vitro studies demonstrated that NDX retention within both cell lines were improved compared to free drug administration. In addition Ki67 and caspase studies demonstrated enhanced cancer cell death mediated by NDX administration compared to free Dox administration. Preclinical retention studies showed that NDX administration in the right lobe of the brain resulted in the presence of Dox at the 72 h time point, while the administration of Dox alone resulted in rapid drug dissipation with no drug presence at 72 h. Importantly, toxicity studies were conducted to examine the presence of edema and swelling in the left lobe of the brain following right lobe administration. While PBS and ND administration in the right lobe resulted in healthy left lobe, unmodified Dox administration into the right lobe resulted in significant swelling in the left lobe, serving as an indicator that free Dox administration could cause major off-target toxicity, even with localized CED administration. It should be noted that NDX administration into the right lobe resulted in healthy left lobe. This was an important finding confirming that Dox could be confined to the tumor-containing regions of the brain via NDX which may markedly improve the tolerance of CED Dox injection (Fig. 5).
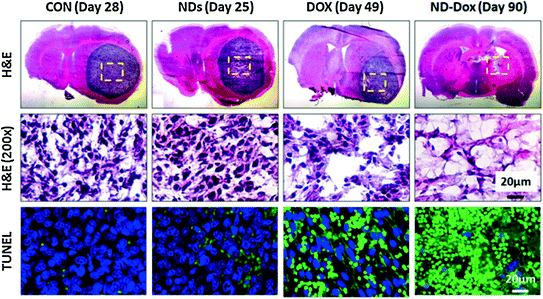

Fig. 5
Nanodiamond-Dox (NDX) administration via CED to treat glioblastoma in a rat model results in tumor regression compared to unmodified drug administration and controls. This was further confirmed via tissue staining and apoptosis assays. Reprinted with permission from Elsevier
Extensive preclinical studies pertaining to NDX efficacy and safety in both C6 and U251MG were conducted. In the U251MG model, tumors observed following PBS and ND administration resulted in animal mortality. Dox administration resulted in an observable reduction in tumor size. However, NDX administration resulted in a significant lengthening of animal survival and cancer cell apoptosis. In the C6 model, the control and Dox-only conditions resulted in largely the same outcomes as those observed in the U251 model. However, NDX administration resulted in tumor regression to the point where the luciferase signal emitted from the tumors were no longer observable and pronounced improvements in animal survival were observed. Hematoxylin and Eosin (H&E) staining showed that the tumors were virtually undetectable, and TUNEL staining revealed significant increases in apoptosis mediated by NDX.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree