Chapter 47 Mycoses Caused by Molds
Infections caused by molds are uncommon but important causes of opportunistic infections in patients with advanced acquired immunodeficiency syndrome (AIDS). The most common fungal infections are due to Candida species and Cryptococcus neoformans. Less commonly seen are the endemic dimorphic mycoses, such as those due to Histoplasma capsulatum and Coccidioides immitis. The least commonly encountered mycoses in patients with HIV are the miscellaneous infections caused by organisms grouped as primary mycelial fungi, or molds.1,2 Since the development of highly active antiretroviral therapy (HAART), infections with molds have become even less common, although mold infections due to an expanding list of organisms continue to occur.3–13 Most often patients have advanced human immunodeficiency virus (HIV) infection with CD4+ T-lymphocyte counts usually less than 200 cells/μL (often less than 50/μL) with uncontrolled HIV viral replication. Other risk factors, such as neutropenia, injection drug use, malignancy, the use of corticosteroids or intravenous catheters, and other chronic medical illnesses, are frequently present.14,15 The clinical presentation of these fungi ranges from sinusitis and soft pulmonary infection to localized deep tissue abscess and widely disseminated disease (Table 47-1). The possibility of a fungal infection must be considered early to allow prompt diagnosis and institution of antifungal therapy and surgical intervention as needed. Long-term suppressive therapy for these mycoses is often required because relapse is frequent. Improved antifungal therapies with excellent activity against molds, particularly the extended spectrum azoles-voriconazole and posaconazole, have been introduced which offer the potential for improved outcomes and less toxicity especially for long-term therapy. Specific guidelines for discontinuing therapy after immunologic reconstitution during HAART have not been developed due to the rarity of these infections, although approaches for the dimorphic pathogen Penicillium marneffei have followed the recommendations in histoplasmosis.16
Table 47-1 Clinical Manifestations of Selected Mold Infections in Patients with AIDS
| Organism | Clinical Manifestations |
|---|---|
| Agents of Hyalohyphomycosis | |
| Aspergillus spp | Pulmonary, sinusitis, cutaneous, focal abscess, disseminated disease |
| Scedosporium apiospermum | Pneumonia, sinusitis, endocarditis, disseminated disease, meningitis |
| Fusarium spp | Fungemia, endocarditis, disseminated infection |
| Chrysosporium spp | Osteomyelitis |
| Trichosporon spp | Catheter-related fungemia |
| Geotrichum spp | Esophageal ulcer, disseminated infection |
| Paecilomyces spp | Disseminated infection |
| Penicillium decumbens and other spp | Disseminated infection |
| Penicillium marnefeii | Disseminated infection |
| Agents of Phaeohyphomycosis | |
| Alterneria spp | Nasal soft tissue infection, sinusitis |
| Exophiala spp | Esophagitis, soft tissue infection |
| Hormonema spp | Liver abscess |
| Cladophialophora bantiana | Brain abscess, pulmonary |
| Phialophora spp | Disseminated infection |
| Bipolaris spp | Endophthalmitis |
| Scedosporium prolificans | Disseminated infection |
| Scopulariopsis | Disseminated infection, brain abscess |
| Agents of Zygomycosis | |
| Rhizopus arrhizus | Orbit, soft tissue, sinus |
| Absidia corymbifera | Renal abscesses, pharyngeal, pulmonary |
| Cunninghamella bertholletiae | Soft tissue abscess |
| Mucor spp | Sinus |
Data from Cunliffe NA, Denning DW. Uncommon invasive mycoses in AIDS. AIDS 9:411, 1995; and Minamoto GY, Rosenberg AS. Fungal infections in patients with the acquired immunodeficiency syndrome. Med Clin North Am 81:381, 1997; and case reports.3,4,11,13,150,151
PATHOGENS AND EPIDEMIOLOGY
The mycologic classification of molds includes dimorphic pathogens (organisms that exist in nature as molds but are seen as yeasts or yeast-like organisms in tissue), agents of hyalohyphomycosis (lightly pigmented molds) or phaeohyphomycosis (darkly pigmented molds), and Zygomycetes (fungi with wide, rarely septated hyphae). This chapter includes the dimorphic fungi Paracoccidioides brasiliensis, Blastomyces dermatitidis, Penicillium marnefeii, and Sporothrix schenckii; agents of hyalohyphomycosis (Aspergillus, Fusarium, Pseudallescheria, Trichosporon, and other less commonly encountered hyaline molds); Zygomycetes (Rhizopus species, and other members of the order Mucorales); and agents of phaeohyphomycosis (Alterneria, Bipolaris, Exophiala, and other dematiaceous molds). Agents of dermatophytosis, including Microsporum gypseum and Trichophyton rubrum, can also cause disseminated infection including invasive soft tissue disease in highly immunosuppressed patients.7,8
Infection by molds most commonly occurs by inhaling conidia (B. dermatitidis, P. brasiliensis, probably P. marneffei, Aspergillus species, Zygomycetes). However, intravenous injection of conidia through injection drug use (Zygomycetes, Aspergillus species) or direct percutaneous inoculation (S. schenckii, agents of phaeohyphomycosis) also occur. After infection, progression or resolution depends on the specific fungal pathogen and the immunocompetence of the patient. Aspergillus, Zygomycetes, and even rare fungal agents of human disease such as Schizophyllum commune (mushroom) have caused infection in the maxillary sinuses of patients with AIDS.17–19
These mycoses generally occur during late stages of AIDS, causing widely disseminated infection. Other risk factors, such as neutropenia, use of corticosteroids, cytomegalovirus infection, and chemotherapy, compound the risk of becoming infected with many molds, such as Aspergillus. Clinical manifestations of these molds are summarized in Table 47-1.
DIAGNOSIS AND THERAPY
Diagnosis for all of these infections depends on direct demonstration of the pathogen by histopathology, culture, or serologic testing (Table 47-2). Serologic testing for antibody may be useful for some mycoses (paracoccidioidomycosis, blastomycosis, and coccidioidomycosis), but such tests may be negative in the severely immunocompromised patient and, if negative, may not be helpful. For histoplasmosis, antigen tests are a primary tool for diagnosis in clinical use. More recently, antigen tests have been developed for paracoccidioidomycosis and for blastomycosis.20–23 Cross reactivity of these antigens with the histoplasma antigen limits the value of antigen testing to discriminate among them.20,24 Detection of glactomannan by ELISA (Platelia EIA, BioRad, Redmond, WA) has been approved for use in invasive aspergillosis, but has not been evaluated extensively in patients with AIDS.25,26 Another nonspecific fungal marker, β-D-glucan, which uses a limulus Amoebocyte lysate assay (Fungitell, Associates of Cape Cod, Falmouth, MA), is commercially available and is positive in molds (but not Zygomycetes, nor Cryptococcus) but has also not been validated in patients with AIDS.27,28 Quantitative PCR testing has been used investigationally for diagnosis of histoplasmosis, blastomycosis, and coccidioidomysosis, as well as aspergillosis.23,29–31 However, primers are not yet standardized or widely available, and PCR for molds, including aspergillosis, remains investigational.32 A culture from any site is usually sufficient to establish a diagnosis of infection because most of these organisms do not usually cause colonization. A major exception is Aspergillus, which may colonize the respiratory tract and not be associated with active infection.33,34 For aspergillosis, the underlying state of host immunity to some degree predicts a benign or invasive course. Other molds, such as Scedosporoium prolificans, occasionally colonize the respiratory tract or sinuses without causing invasive disease.35 The diagnosis of invasive infection should be considered in patients with a compatible clinical syndrome.
Table 47-2 Characteristics of Dimorphic Mycoses in Patients with AIDS
| Mycosis | Geographic Location | Frequency/Form |
|---|---|---|
| Histoplasmosis | US/Latin America | 30% HIV local/disseminated infection, reticuloendothelial system |
| Coccidioidomycosis | Southwest US/Latin America | Locally common/primary and reactivation |
| Blastomycosis | US/scattered worldwide | Rare/pulmonary, disseminated |
| Paracoccidioidomycosis | South America | Rare/disseminated |
| Penicilliosis | Southeast Asia | Local 20% HIV/disseminated, pulmonary and skin |
| Sporotrichosis | Scattered worldwide | Uncommon/cutaneous, disseminated disease |
Treatment of the invasive molds has evolved considerably in recent years. Until recently amphotericin B has been the unchallenged standard therapy for most of these organisms. Major recent developments have increased therapeutic options which have changed the drug of choice for several of the major mold pathogens. Chief among these newer agents has been voriconazole, a potent very broad spectrum triazole, which was found to be clearly superior to amphotericin B in treatment of invasive aspergillosis.36 Voriconazole has also been found highly efficacious in treatment of phaeohyphomycosis, and may be considered the drug of choice for these infections.37 Voriconazole is very well absorbed (>90%) and penetrates the cerebrospinal fluid at 60% of the plasma concentration.38 Voriconazole also has some efficacy in fusariosis and against Scedosporium apiospermum. These uncommon infections, when localized, tend to respond to voriconazole, but are less responsive in widely disseminated infection.37 Voriconazole has no efficacy against pathogens causing zygomycosis, but these are extremely rare in patients with AIDS. Posaconazole is another broad spectrum triazole licensed in Europe. The antifungal spectrum is similar to that for voriconazole, but whose spectrum of activity includes Zygomycetes.39–43 There is less experience with posaconazole, although there appear to be somewhat fewer drug interactions than voriconazole and possibly less hepatic toxicity, due to its lack of hepatic metabolism.44 Posaconazole is presently recommended as a salvage drug due to the fact it exhibits saturable absorption and is not yet available in an intravenous formulation.45 Activity of posaconazole in salvage therapy of zygomycosis has been particularly encouraging.39,43 There are few clinical data on either voriconazole or posaconazole for the less common dimorphic mycoses. The utility of an earlier azole, itraconazole, is limited for molds because of erratic bioavailability and toxicity.46 The solution formulation of itraconazole improves bioavailability but there are limited data to support its use in serious mold infections, although data support the use of itraconazole in dimorphic pathogens.47–50 Lipid formulations of amphotericin B have largely replaced amphotericin B deoxycholate for mold infections. A recent trial comparing high (10 mg kg−1 day−1) with low (3 mg kg−1 day−1) liposomal amphotericin B as initial therapy suggests that the high dose added nothing to the efficacy and was somewhat more toxic.51 The echinocandins (e.g., caspofungin, micafungin, anidulafungin) have efficacy in candidiasis, for which these agents are fungicidal, but against molds they only inhibit growing Aspergillus mycelia.52 Thus, these agents are used as salvage therapy for invasive aspergillosis although only caspofungin has regulatory approval for that indication.53
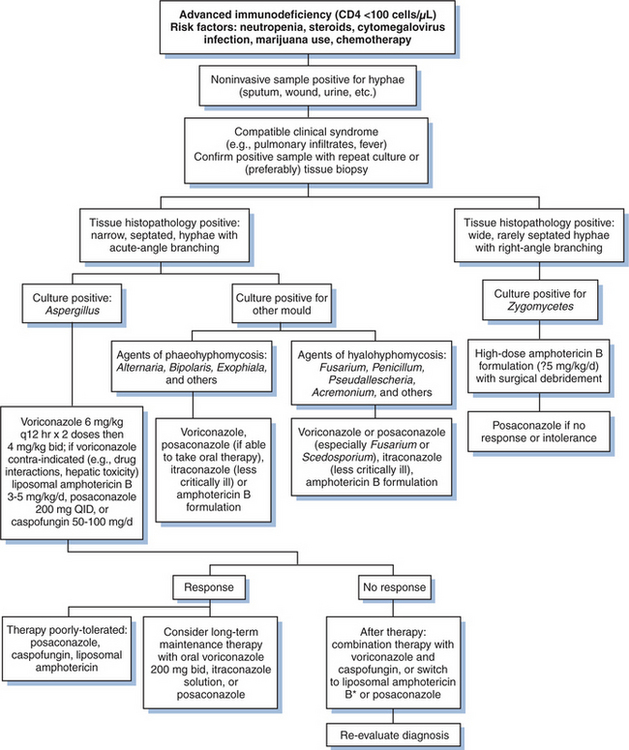
Although few randomized trials for molds have been conducted, guidelines for managing fungal infections (though mostly in non-HIV-infected patients) have been published.48,54–58 An approach to therapy is summarized in Figure 47-1. Most important is a high index of suspicion for the diagnosis, particularly in patients with risk factors for invasive infections (e.g. steroid use, neutropenia, chemotherapy). Positive cultures from noninvasive samples (e.g., sputum, wounds, urine) may indicate infection, particularly if they are repeatedly positive. Bronchoalveolar lavage and, when appropriate, tissue biopsies should be undertaken to confirm the presence of hyphae. Culture specimens should be obtained to confirm the specific mold, as treatment options may vary.
Because aspergillosis is the most common of the filamentous pathogens, antifungal therapy with high-dose voriconazole should be initiated promptly in most patients with life-threatening disease. Voriconazole should not be used when the pathogen is an agent of zygomycosis (a rare event). Amphotericin B is an alternative, generally used at 3–5 mg/kgof liposomal amphotericin B. Exceptions are patients known to be infected with an amphotericin B-resistant pathogens such as Aspergillus terreus or Scedosporium apiospermum.37,40,59 Adjunctive surgical resection of isolated lesions (as may occur with Aspergillus when lesions are juxtaposed to the pulmonary artery) or débridement of infection caused by Zygomycetes may improve outcome. Following initial therapy, long-term suppressive therapy with an oral azole is warranted for most mold infections. Long-term survival with many of these infections is uncommon unless the HIV-induced immunosuppression is corrected.
“DIMORPHIC” MYCOSES (SPOROTHRIX, PENICILLIUM, BLASTOMYCES)
Pathogen
The dimorphic mycoses are caused by fungi that are pathogenic to normal and immune hosts. These organisms tend to occur in certain limited geographic areas or have specific epidemiologic niches (Table 47-3). The mycoses caused by these organisms include those due to endemic fungi, histoplasmosis, coccidioidomycosis, paracoccidioidomycosis, blastomycosis, penicilliosis (caused by the fungus Penicillium marneffei), and sporotrichosis. Among the agents of dimorphic mycoses, Sporothrix schenckii does not occur in a specific geographic zone but does occur in the specific setting of exposure to contaminated vegetative material such as moss and rosebush thorns. All are dimorphic; that is, they have an infectious mycelial form in nature and a yeast or yeast-like parasitic form in humans. Histoplasmosis and coccidioidomycosis are discussed in Chapters 44 and 45, respectively.
Table 47-3 Diagnosis of Mold Infections in Patients with AIDS
| Organism | Tissue Characteristics | Serology/Others |
|---|---|---|
| Agents of hyalohyphomycosis | ||
| Aspergillus spp | Septated, acute-angle branching | Antibody not useful; galactomannan detection; PCR investigational; β-D-glucan nonspecific fungal marker; blood cultures usually negative |
| Other agents | Indistinguishable from | Serology not available for molds other than Apsergillus |
| Aspergillus spp | Fusarium may grow from blood cultures | |
| Agents of phaeohyphomycosis | Irregular hyphae, confirm melanin with specific | Serology not available |
| Masson–Fontana stain | ||
| Agents of zygomycosis | Wide, rarely septated hyphae | Serology not available; may not grow from homogenized tissues |
The infectious particle is the conidium, or spore. All of the dimorphic pathogens may infect by inhalation, although Sporothrix infection often is due to percutaneous inoculation.60 Within a short time after infection occurs, the conidia are ingested by macrophages or monocytes and within hours to days the fungus converts to the parasitic form. Infection is ultimately controlled by cell-mediated immune responses. However, in patients with HIV infection, T-helper cell (TH2) responses are depressed or ablated. The fungus may grow and spread locally or hematogenously without much restriction. In patients with AIDS the endemic mycoses are more frequently disseminated compared to their presentation in non-HIV-infected hosts. The clinical manifestations of these diseases in HIV-infected patients are summarized in Table 47-1. The geographic distributions and clinical forms are summarized in Table 47-2.
Some infections caused by dimorphic pathogens remain uncommon in patients with AIDS. For example, paracoccidioidomycosis is the major endemic mycosis in South America, but infection in patients with AIDS is rare.11,57 When the disease does occur in AIDS patients, its presentation is distinct from that seen in normal hosts. In immunocompetent subjects paracoccidioidomycosis is associated with chronic progressive pulmonary granulomatous disease and dense fibrosis progressing over years. In patients with AIDS there is much more rapid progression, and the disease closely resembles disseminated histoplasmosis, with mucocutaneous skin lesions, miliary pulmonary infiltrates, and adrenal insufficiency being significant components. Meningitis may also occur, but fibrosis is not a major component of this form of disease. Blastomycosis is also uncommon in AIDS patients, occurring mainly as a late complication in those with advanced AIDS.61,62 In immunocompetent hosts blastomycosis is associated with pulmonary fibrosis or skin infection; in patients with AIDS, however, there is widely disseminated infection, including meningitis.63,64
In contrast, penicilliosis caused by the dimorphic fungus P. marneffei has become the major endemic fungus of Southeast Asia, appearing particularly in southern China and northern Thailand.12,57,65–67 Penicilliosis may occur in immunocompetent patients but more commonly is seen as an opportunistic infection of AIDS. Multisystem involvement with pulmonary, skin, and visceral lesions is similar in AIDS and nonAIDS patients. Penicilliosis is a febrile wasting disease associated with symptoms that mimic other granulomatous diseases such as tuberculosis or histoplasmosis. There are commonly seen cutaneous lesions with umbilicated centers. These lesions resemble molluscum contagiosum. Like Histoplasma, P. marneffei may be recovered in blood cultures; however, in contrast to H. capsulatum, the yeasts of Penicillium divide by fission rather than by budding, so a central zone of clearing between the yeasts may be observed.
Sporotrichosis is most commonly associated with lymphocutaneous disease in immunocompetent patients.68 However, infection may also result from inhalation of conidia, so pulmonary infection and disseminated disease may occur. Widespread disease involving joints and the lungs and other tissues is more common in HIV-infected patients. Meningitis, which is usually rare in sporotrichosis, has been reported in a few patients with HIV infection.9,60,69 Untreated, these pathogens may spontaneously resolve in immunocompetent patients; but in patients with immunodeficiency such as AIDS, these mycoses are commonly progressive and lethal.
Diagnosis
Table 47-3 summarizes diagnostic features of mold infections.
The fungi causing all of these dimorphic mycoses exist in nature in their mycelial forms. This is presumably related to nutritional requirements and, for B. dermatitidis and P. marneffei, may be related to animal vectors of disease (beavers and, presumably, bamboo rats, respectively). Paracoccidioides brasiliensis is found in certain moist tropical forest areas, but its natural habitat or vector within those zones is unclear. It has been associated with agricultural occupations (including farming) and gold mining. Sporothrix schenckii is also associated with outdoor activities, particularly trauma from garden exposures and sphagnum moss. Although sporotrichosis is found as far north as Canada, a number of small hyperendemic zones have been seen in tropical countries, where this infection is more common than in temperate climates.70
It is assumed that nutritional requirements also in large part dictate the endemic zones of these fungi. The endemic zone for blastomycosis in North America and the zone for paracoccidioidomycosis in South America largely overlap the endemic zones for histoplasmosis. However, relatively few cases of blastomycosis or paracoccidioidomycosis are associated with AIDS, whereas histoplasmosis is a major pathogen in AIDS patients. The clear rural associations of infection with B. dermatitidis or P. brasiliensis are offered as a contrast to histoplasmosis, which is seen in both urban and rural settings and is associated with construction. Late reactivation of disease appears to be less common with paracoccidioidomycosis than histoplasmosis. Another factor that may play a role in “suppressing” paracoccidioidomycosis is the frequent use of trimethoprim–sulfamethoxazole (TMP-SMX) for preventing pneumocystosis. TMP-SMX is active against P. brasiliensis as well, so a secondary benefit of preventing P. brasiliensis is theoretically possible.71
These dimorphic pathogens are identified by culture or presumptively by identifying distinct tissue forms on tissue samples. B. dermatitidis, S. schenckii, and P. marneffei grow readily as mycelia on usual fungal media such as Sabouraud dextrose agar. P. marneffei produces a distinctive red pigment that diffuses out into the medium and facilitates identification. Mycelial cultures may take up to a month to grow at room temperature. P. brasiliensis grows slowly in the mycelial phase and requires a temperature range of 18–22°C and McVeigh Morton agar to grow optimally.71
Cultures may be prepared from sputum, cutaneous lesions, lymph nodes, or urine. Blood can be cultured by the isolator technique or using standard blood culture systems. Culture may require up to a month for growth to be detected, and it is sometimes falsely negative because of failure of the organism to convert from the parasitic form to the mycelial form in vitro. It is much more rapid and feasible to identify the organism in tissue using the Gomori methenamine silver stain or periodic acid-Schiff, each of which stains the cell walls. The presence of these organisms on tissue biopsy, wet mount, or culture is indicative of infection and may be associated with pulmonary or disseminated disease.72
Serologic testing is less useful than with histoplasmosis (which can be diagnosed with antigen testing) or coccidioidomycosis (for which positive antibody results correlate with the disease). Antibody tests, and more recently antigen tests, are available for paracoccidioidomycosis and blastomycosis.20,22,30,57,73,74 Although the antibodies measured can be useful for diagnosis and their titers tend to rise with worsening disease, they are not protective. There is some cross reaction (especially for B. dermatitidis) with H. capsulatum and C. immitis.20,73 There is a serologic test for sporotrichosis, but it is not widely used. A serologic test has been developed for P. marneffei disease but is still not used routinely.75 Antibody testing may be less useful in patients with HIV infection, particularly during the late stages when antibodies are poorly produced. Fungal antibody tests are routinely available through clinical or reference laboratories. Antigen testing is clinically available only for histoplasmosis and blastomycosis (Histoplasmosis Reference Laboratory, Indianapolis, IN, USA).
Susceptibility testing of the endemic mycoses remains nonstandardized. In general, modification of the standard yeast susceptibility testing method of the Clinical Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) is used, but incubation is carried out for longer periods. As a practical tool, susceptibility testing has been correlated with clinical response for P. marneffei, and for H. capsulatum for which amphotericin B, itraconazole, posaconazole, and to a lesser extent voriconazole are more potent in vitro than fluconazole, with corresponding clinical results.76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree