Chapter 41 Mycobacterium Avium Complex and Other Atypical Mycobacterial Infections
CLINICAL SYNDROMES
Mycobacterium avium complex (MAC) disease in patients with human immunodeficiency virus-1 (HIV-1) infection with acquired immunodeficiency syndrome (AIDS) and advanced immunosuppression, in the absence of potent antiretroviral therapy, is generally associated with disseminated multiorgan infection. Early symptoms may be minimal and may precede detectable mycobacteremia by several weeks.1,2 Although mycobacteremia may be intermittent, transient, or absent, untreated disease in most patients with advanced immunosuppression progresses to clinically apparent symptoms, sustained bacteremia, and widespread infiltration of reticuloendothelial and organ tissue.1–7 Those with established blood and widespread tissue infection report symptoms that include fever, drenching sweats, weight loss, fat and muscle-wasting, fatigue, diarrhea, and abdominal pain.1,3–6 Localised MAC disease, while uncommon, is more often seen among patients with advanced immunosuppression who have a substantial increase in the CD4+ T-lymphocyte count (100 cells/μL or higher) and suppression of HIV-1 replication after initiation of potent combination antiretroviral therapy.8–10 Focal lymphadenitis and fever, or other localized tissue infections caused by MAC, unaccompanied by bacteremia or disseminated tissue or organ involvement, have been described. Similarly, an inflammatory response that mimics active MAC disease may occur in those with previously established but quiescent or treated tissue infection and rapid and substantial increases in CD4+ T-lymphocyte counts after starting potent antiretroviral therapy.8–10 Signs and symptoms of this immune recovery inflammatory syndrome may be clinically indistinguishable from those of active MAC infection but are generally unaccompanied by detectable mycobacterial replication or growth in culture or tissue samples. In patients not receiving, or who have not immunologically responded to, potent combination antiretroviral therapies, localized disease is rare. The localized syndromes described in HIV-1-infected individuals include a diarrheal malabsorption syndrome resembling Whipple’s disease; cervical or mesenteric lymphadenitis; pneumonitis; pericarditis; osteomyelitis; skin, soft tissue or muscle abscesses; renal lesions; an enlarging genital ulcer; or central nervous system infection (CA Benson, personal observations).4,11–21 Phillips and colleagues estimated the incidence of immune reconstitution inflammatory syndromes due to MAC to be 3.5% among patients with CD4+ T-lymphocyte counts less than 100 cells/μL who initiated potent combination antiretroviral therapy. The three most common presentations in their series of 45 patients included peripheral lymphadenitis, pulmonary thoracic disease and intraabdominal disease.22 More recent reports suggest that rates of immune recovery inflammatory syndromes may be as high as 31% among patients with preexisting MAC infections who were started on and responded to potent combination antiretroviral therapy.21
The most frequently observed laboratory abnormalities in persons with disseminated M. avium disease are anemia, elevated liver alkaline phosphatase, and decreased serum albumin.3–623 Neutropenia or thrombocytopenia (or both) may accompany bone marrow infiltration. Hepatomegaly, splenomegaly, or central (paratracheal, retroperitoneal, or para-aortic) lymphadenopathy may be detected on physical examination or by radiographic or other imaging studies.3,4,24 With the exception of focal lymphadenitis, peripheral lymphadenopathy is uncommon. Other focal symptoms, signs, or laboratory abnormalities may occur in the context of the localized disease syndromes previously described.
PATHOGEN
The M. avium complex is comprised of two predominant species, M. avium and M. intracellulare, and several unspeciated mycobacteria.3,25 MAC organisms are considered nonphotochromogenic, although production of yellow pigment by older colonies grown under select conditions has been reported.26 Growth on solid media is generally slow, but growth can be detected radiometrically in liquid media within 7–10 days depending on inoculum size and laboratory conditions.27 The colonial morphotype on solid media is determined by the glycopeptidolipid content of the cell wall, the characteristic that distinguishes serovars. Of the more than 28 identified serovars, types 1–6, 8–11, and 21 are M. avium, and types 7, 12–20, and 25 are M. intracellulare.26,28 Types 1, 4, and 8 of M. avium are most frequently associated with disease in persons with AIDS in the United States and developed countries.26,28
Previous studies have shown that more than 95% of isolates of MAC recovered from patients with AIDS and disseminated MAC disease are M. avium.29–33 In contrast, 40% of MAC isolates from HIV-uninfected individuals are M. intracellulare.32 Characteristics of individual serovars and unique host genetic or immune factors may determine differences in the prevailing organisms causing disease in different hosts or geographic regions.34
Up to 25% of persons with AIDS and disseminated M. avium disease may be infected with two or more genetically distinct strains of M. avium.35–37 The clinical significance of this finding is unknown, although in one study different strains recovered before and during treatment demonstrated disparate susceptibility to antimicrobial agents.35–37 In one recent study, differences in virulence factors (as measured by invasion of gastrointestinal cells and replication in macrophages) of MAC isolates obtained from mucosal surfaces and from blood suggested that these factors may contribute to the pathogenesis of MAC infection in immunocompromised hosts.38
EPIDEMIOLOGY
MAC organisms can be recovered from a number of environmental reservoirs, including water, soil, domestic and farm animals, birds, foods, and some tobacco products.33,39,40 In early studies evaluating delayed-type hypersensitivity responses to skin testing with purified protein derivative, Battey demonstrated a high prevalence of responses among persons residing in the southeastern part of the United States.41 Studies using a more specific mycobacterial antigen derived from M. avium, sensitin, suggest that 7–12% of adults in the United States, Kenya, Trinidad, and Finland have been previously exposed to or infected with M. avium.42 Although environmental reservoirs of M. avium also exist in developing countries or regions, MAC disease is uncommon among patients with AIDS in these areas.42,43 Hypotheses suggested to explain this observation are that the high prevalence of latent or active M. tuberculosis infection in developing countries confers partial immunity to atypical mycobacterial infection or that biologic characteristics among local environmental serovars of MAC diminish their virulence.4,43,44
The environmental source of infection for individuals who develop active disease, with rare exception, is indeterminate. An epidemiologic study of MAC isolates from HIV-infected patients in Japan showed no evidence of clustering of similar isolates among patients.45 In contrast, von Reyn et al described two small clusters of HIV-infected individuals with disseminated MAC disease from whom MAC isolates were recovered and found to be genetically identical to isolates recovered from potable water supplies in the same hospitals in which these patients had previously been hospitalized.46 Household water supplies have also been shown to be ‘contaminated’ with environmental strains of M. avium.47 Von Reyn and colleagues further reported an association between ingestion of raw fish, prior bronchoscopy, or treatment with granulocyte colony-stimulating factor and an increased risk of developing disseminated MAC disease.48 Similarly, Horsburgh et al demonstrated an association between ingestion of hard cheese and an increased risk of developing MAC bacteremia.49 In the latter two studies an association was also noted between daily showering or occupational exposure to water and a decreased risk of developing MAC bacteremia.48,49 As these studies illustrate, however, no environmental exposure or behavior has been consistently associated with the subsequent development of MAC disease in susceptible persons; therefore behaviors aimed at avoidance of exposure cannot be expected to result in a decreased risk of developing disease for highly susceptible individuals.50–52
Prior to the advent of effective chemoprophylaxis and potent combination antiretroviral therapy, the incidence of disseminated MAC disease in persons with AIDS ranged from ∼20% to 40%.5,6,53,54 There has been a dramatic decline in the incidence of opportunistic infections overall and specifically in M. avium disease during the modern era of potent combination antiretroviral therapy. The clinical event rate in one study comparing two drugs for prophylaxis of Pneumocystis jerovici pneumonia in subjects intolerant of trimethoprim-sulfamethoxazole declined from 64 per 100 patient-years during the first year of follow-up to 34 per 100 patient-years during the second year; the proportion of study subjects who used protease inhibitor (PI) therapies increased from none at the beginning of the first year to 72% at the end of the second year of the study.55 In the Centers for Disease Control and Prevention (CDC)-sponsored HIV Outpatient Study (HOPS), the overall proportion of persons followed with AIDS who had CD4+ T-lymphocyte counts of less than 100 cells/μL and who developed an opportunistic infection in 1995 was 26.9%, compared with 18.1% in 1996; the rate declined from 4.8% during the fourth quarter of 1996 to 3.0% during the second quarter of 1997 coincident with the increased use of PI therapy.56 Although patients with low CD4+ T-cell counts remain at risk for MAC, among patients in the Johns Hopkins cohort with advanced HIV disease the proportion developing MAC has fallen from 16% before 1996 to 4% after 1996 with a rate of less than 1% in 2003.57 Factors associated with developing MAC in the Hopkins cohort include younger age, no use of potent combination antiretroviral therapy, and enrollment before 1996. Similar rates of decrease of opportunistic infections were reported by investigators following implementation of PI therapies in Europe.58,59 Additional studies have indicated that rates of M. avium disease in similar populations have declined in concert with the rates of other opportunistic infections.60–62 Similarly, in two randomized clinical trials evaluating azithromycin or placebo for prophylaxis of MAC disease in patients on potent combination antiretroviral therapy who had an increase in their CD4+ T-lymphocyte counts from less than 50 cells/μL to more than 100 cells/μL at entry, the overall rate of MAC disease was fewer than two cases per 100 patient-years of follow-up.61,62 These rates are considerably lower than those reported for previous years in the absence of potent antiretroviral therapy and chemoprophylaxis.
The increased susceptibility of HIV-infected persons with advanced immunosuppression to disseminated M. avium disease has not been fully explained. CD4+ and CD8+ T-lymphocytes, macrophages, natural killer (NK) cells, gamma delta T cells, and the host of cytokines produced by these cells in response to mycobacterial antigen stimulation represent the primary immune response to infection with MAC organisms.63–74 Among the cytokines most central to the host immune response to MAC appear to be interleukin-12 (IL-12), interferon-γ (IFNγ), tumor necrosis factor-α (TNFα), IL-6, and IL-10.63–74 Animal studies show that TNFα is essential for the development of protective immunity to MAC and contributes to the reduction of intracellular growth of MAC.75,76 IL-12 stimulates IFNγ production, and both play key roles in the immune defense against MAC disease. In mice lacking these cytokines after targeted gene deletions and in humans with genetic defects of IL-12 or IFNγ production or expression, increased susceptibility to MAC infection and severe forms of disseminated MAC disease ensue.74,77–82 IL-6 promotes M. avium growth, and IL-10 appears to inhibit production of TNFα and IL-12; IL-10 also down-regulates expression of co-stimulatory molecules on M. avium-infected monocytes, resulting in inhibition of IFNγ production.74,83–86 Whereas in vitro macrophage function and response to IFNγ and the induction and expression of TNFα appear to be similar in HIV-infected individuals and HIV-uninfected controls, both HIV-infected and HIV-uninfected individuals with established MAC disease have been reported to have increased expression of IL-6, decreased production or expression of IFNγ and IL-12, and increased production of IL-10 by peripheral blood mononuclear cells.71–74,87,88 These data suggest that, in addition to CD4+ T-lymphocyte depletion and loss of CD4+ T cells, perturbations of these cytoki-nes are involved in the increased propensity of HIV-infected individuals to develop disseminated MAC disease. Functionally as well, untreated HIV-infected patients have significantly reduced lymphoproliferative responses to M. avium.73
MacGregor and colleagues examined immunologic factors associated with development of MAC among patients with advanced immunosuppression with and without signs and symptoms of MAC disease.89 Subjects with disseminated MAC and asymptomatic controls with CD4+ T-lymphocyte counts of less than 50 cells/μL had similar levels of measured cytokine producing cells in bone marrow tissue including a low frequency of cells producing IL-12, TNFα, and TGF-β, however, significantly more CD8+ T cells were present among those with MAC infection compared to controls. In contrast, the group with MAC infection had significantly more CD4+ T-lymphocytes secreting IFNγ in response to MAC antigen and lower numbers of CD8+ T cells in peripheral blood than did the control group. These results suggest that ineffective MAC specific T-cell responses can persist during MAC infection. The authors speculate that the inflammatory responses to MAC that can occur shortly after initiation of potent combination antiretroviral therapy might be due to the rapid expansion of these preexisting immune responses.89 A recent observation of a dramatic increase from baseline in PPD-specific Th1 IFNγ-producing CD4+ T cells during immune recovery inflammatory syndromes in patients with tuberculosis support this hypothesis.90
The most important risk factor for or predictor of the development of disseminated M. avium disease is advanced immunosuppression, as indicated by a CD4+ T-lymphocyte count of less than 50 cells/μL.51–56,58–62 This remains true in patients treated with potent antiretroviral therapy.58–62 High plasma HIV-1 RNA levels (>100 000 copies/mL) and a prior opportunistic infection, particularly cytomegalovirus disease, also contribute to the hazard of developing M. avium disease.53,54,91–94
Data regarding the relative risk of developing a specific opportunistic infection in those receiving potent combination antiretroviral therapies are limited, but as previously described, the risk has declined substantially, attributed not only to increases in CD4+ T-lymphocyte counts in response to therapy but also to more effective control of HIV-1 replication. For example, in the DACS 071 study, a retrospective analysis of the risk of developing specific opportunistic infections in patients who participated in four prospective randomized ACTG antiretroviral treatment trials, the risk of developing disseminated M. avium disease was reduced threefold at 24 months for those who experienced even a modest decline in the plasma HIV-1 RNA level of 0.5 log10 copies/mL 8 weeks following initiation of combination antiretroviral therapies that included nucleoside or non-nucleoside reverse transcriptase inhibitors (NNRTIs) (or both).93 In an evaluation of the relationship between viral load and the occurrence of MAC disease (among other opportunistic infections), investigators from the Multicenter AIDS Cohort Study (MACS) showed that the relative hazard of developing M. avium disease for those with a baseline HIV-1 RNA level of 30 000–60 000 copies/mL was 1.29; it increased to 9.85 for those with a baseline HIV-1 RNA level of 60 000–90 000 copies/mL and to 14.97 for those with a baseline HIV-1 RNA of more than 90 000 copies/mL.94 HIV-1 RNA level and CD4+ T-lymphocyte count were independent predictors of risk. In the ACTG 320 trial, significant predictors of a shorter time to development of MAC disease for those treated with combination antiretroviral therapy were a 1 log10 copies/mL increment in baseline HIV-1 RNA with at least a 0.5 log10 copies/mL decrease in HIV-1 RNA at week 8 of therapy, as well as a less than 10 cell increase in CD4+ T-lymphocyte count in response to antiretroviral therapy by week 8.60
Aside from immunologic perturbation and the magnitude of HIV-1 replication, other physiologic factors that contribute to the risk of developing M. avium bacteremia and disease include colonization of the respiratory or gastrointestinal tract. Acid-fast bacilli (AFB) smears and cultures of stool or sputum may demonstrate the presence of M. avium prior to detection of mycobacteremia in up to 25–33% of HIV-infected individuals who subsequently develop disseminated M. avium disease.95,96 Colonization of the gastrointestinal or respiratory tract, when detected, appears to have a positive predictive value approaching 60% in a profoundly immunocompromised patient population. In the ACTG 341 study of MAC pathogenesis, respiratory or gastrointestinal colonization was present in 36% of patients with disseminated infection, but in only 5% of noninfected controls with CD4 counts below 50 cells/μL.89 However, the utility of screening cultures of these sites to detect colonization is limited because most patients are not colonized. Furthermore, when colonization can be demonstrated, it may be transient and not followed by the development of disseminated disease.89,95,96
Most prospective studies have not suggested differences in the incidence of M. avium disease according to gender, racial or ethnic group, age, or geographic distribution.5,50,53,54,91,97 However, data collected among homosexual or bisexual men in the MACS indicated that those living in Baltimore (6.9%) or Los Angeles (5.6%) had a higher incidence of MAC disease than those in Chicago (2.6%) or Pittsburgh (0%).91 In another study evaluating the geographic and seasonal variation in M. avium bacteremia among patients with AIDS receiving placebo in a randomized clinical trial of M. avium prophylaxis, those with the highest risk were patients in south central regions of the United States, whereas those with the lowest risk resided in northern states and Canada.98 There also appeared to be a trend toward decreased numbers of cases in northern states during summer months compared to other seasons.
TRANSMISSION
The mode of transmission for M. avium infection is thought to be inhalation, aspiration, or ingestion via respiratory or gastrointestinal tract portals of entry. Support for this contention includes laboratory studies demonstrating that M. avium can be experimentally aerosolized from environmental sources, and that organisms recovered from environmental aerosols are genetically similar to clinical isolates.99,100 Data that support the gastrointestinal route as the predominant portal of entry for HIV-infected patients include the not-infrequent finding of extensive infiltration of the gastrointestinal tract and intraabdominal organs in those with disseminated disease and the finding that disseminated disease rapidly follows intraoral or intrarectal challenge of the beige mouse or immunosuppressed murine or rat models with M. avium.95,96,101–104 Household or close contact of those with M. avium disease are not at increased risk of developing disease, and isolates recovered from individuals, in general, do not appear to be genetically related, indicating that person-to-person transmission is unlikely.35,49 However, at least one study has demonstrated multiple clusters of two or three patients infected with four genetically unique strains of M. avium; the investigators concluded that their data suggested a common environmental source rather than person-to-person spread.37
DIAGNOSIS
Disseminated M. avium disease is diagnosed when a compatible clinical syndrome is present coupled with the recovery of M. avium from cultures of blood, bone marrow, or other normally sterile tissue or body fluids. The disease may be asymptomatic or only mildly symptomatic early; in this setting low-level mycobacteremia may produce intermittently positive blood cultures, although a small proportion of patients may have extensive infiltration of bone marrow or other reticuloendothelial organs in the absence of mycobacteremia.1,3,89,105,106
ISOLATION OF MYCOBACTERIUM
Use of an Isolator (Wampole Laboratories, Cranbury, NJ, USA) or similar blood culture system and subsequent inoculation of blood into Bactec 12B liquid medium or direct inoculation of specimens into Bactec 13A bottles (Bactec; Becton-Dickinson, Sparks, MD, USA) followed by radiometric detection of growth are the most frequently employed methods for isolating M. avium from blood.107 Inoculation onto solid media (e.g., Middlebrook 7H10/11 or Lowenstein–Jensen media) is most useful when quantification of mycobacteria is necessary, but adequate growth requires longer incubation. Species-specific DNA probes, high-performance liquid chromatography, or biochemical tests can be used to identify such organisms as M. avium, M. intracellulare, or other mycobacteria. Polymerase chain reaction assays for the detection of MAC species in clinical samples have not reached a level of sensitivity requisite for diagnostic clinical use.107–109 Depending on the size of the inoculum, the average time required to cultivate and identify M. avium using liquid media, radiometric detection of growth, and DNA probe identification generally ranges from 7 to 14 days.27,110,111
Other ancillary studies that may be useful for diagnosing disseminated MAC disease for HIV-infected patients at risk include AFB smears or stool cultures, biopsies of clinically suspect tissues or organs, radiographic imaging of the abdomen or mediastinum to detect lymphadenopathy, or other studies aimed at recovery of organisms from focal infection sites. The yield of these procedures is generally dependent on the presence of symptoms, signs, or laboratory abnormalities related to the involved sites. The finding of MAC in sputum or stool in HIV-infected individuals may represent colonization rather than disease. Treatment should not be initiated based on these findings alone unless there is other evidence to indicate the presence of active disease.
SUSCEPTIBILITY TESTING
Testing M. avium for susceptibility to antimicrobial agents can be accomplished using conventional agar proportion, agar dilution, broth dilution, or radiometric broth macrodilution techniques. Each may produce differing results depending on the assay and laboratory conditions used. The most widely recommended method for use in clinical laboratories in the United States is radiometric broth macrodilution utilizing Bactec technology.112 The technical performance of assays using this method has been described in detail elsewhere.107,112,113
The use of susceptibility test results to guide selection of initial regimens for treatment of M. avium disease is not uniformly adopted by all clinicians, but most would agree that such testing should be performed on clinically significant isolates recovered from tissue or blood from patients who have received or are receiving azalide or macrolide agents for therapy or chemoprophylaxis. However, the rate of background resistance may be high regardless of prior macrolide treatment. A recent study documented macrolide resistance in 17% of MAC isolates from patients with AIDS and limited or no prior macrolide exposure.114
In early published studies of treatment for pulmonary MAC disease in nonimmunocompromised patients, treatment with four to six drugs guided by susceptibility test results was reportedly associated with improved clinical outcome.115 In clinical trials of patients with AIDS, a relationship between pretreatment susceptibility test results and the microbiologic and clinical responses has been demonstrated primarily for clarithromycin, azithromycin, and, less compellingly, rifabutin.116–118 Relapses caused by clarithromycin- or azithromycin-resistant isolates have been reported when these agents are used as monotherapy during prophylaxis or treatment of M. avium disease, although relapse also occurs in those receiving multiple drug therapy, presumably as a result of poor adherence, poor absorption, the use of ineffective companion drugs, or drug–drug interactions. Thus far evidence indicates that M. avium resistance to clarithromycin is due to single-step mutations in the V domain of the 23S rRNA gene; these mutations confer cross-resistance to azithromycin and probably other macrolides.119,120
Clinical breakpoints for the interpretation of susceptibility test results have been proposed for clarithromycin, azithromycin, and rifabutin based on data from prospective clinical trials.107,112 Minimum inhibitory concentrations (MICs) of 32 μg/mL or more for clarithromycin or 256 μg/mL or more for azithromycin are the suggested thresholds for determining resistance based on the Bactec method for radiometric susceptibility testing.107,112 It has been further suggested by some that the criteria for resistance should be based on the peak concentration achieved in serum after administration of therapeutic doses of drugs; however, clarithromycin, azithromycin, and rifabutin readily penetrate cells and tissue, often to levels far exceeding the MIC of most susceptible M. avium organisms. M. avium survives and replicates within macrophages, where these levels may be highest. This point should be considered when establishing resistance breakpoints or determining the clinical significance of susceptibility test results or serum or plasma concentrations of antimycobacterial drugs.
TREATMENT OF M. AVIUM DISEASE
Initial Therapy
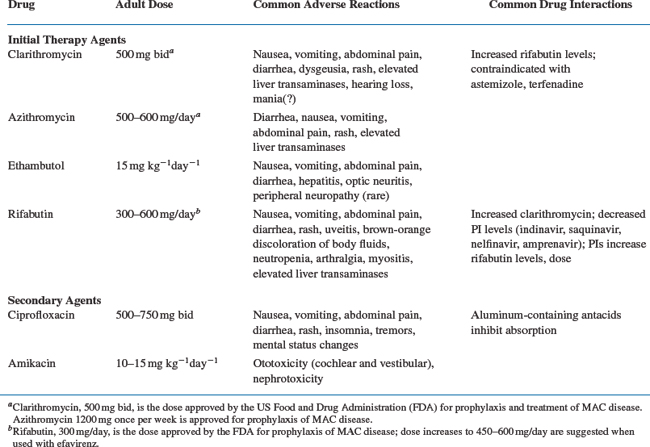
Available antimicrobial agents with demonstrated activity in human clinical trials, alone or in combination regimens, for the treatment of M. avium disease include clarithromycin, azithromycin, rifabutin, ethambutol, and possibly ciprofloxacin and amikacin.1,4,116,121–132 These agents, their commonly recommended adult doses, and their most common side effects are summarized in Table 41-1.3,4,121,133–138
Table 41-1 Antimycobacterial Drugs Suggested for Treatment of Disseminated Mycobacterium Avium Disease in Patients With AIDS
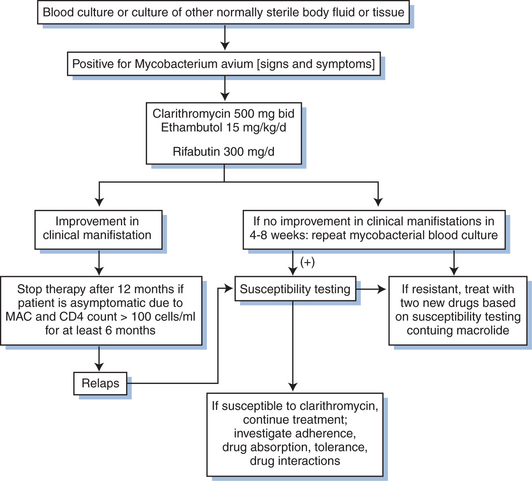
Initial treatment of M. avium disease consists of a combination of at least two antimycobacterial drugs to prevent or delay the emergence of resistance.52,138 Clarithromycin or azithromycin is the preferred first agent, although data regarding the efficacy of azithromycin are more limited than for clarithromycin.52,138 Ethambutol is the recommended second drug. A third or fourth drug selected from among rifabutin, ciprofloxacin, or parenteral amikacin may be added for those with more severe or extensive symptoms or disease.3,4,121,127,133 Alleviation of fever and a decline in the quantity of mycobacteria in blood or tissue can be expected within 2–4 weeks after initiation of appropriate therapy; for those with widespread tissue involvement or high levels of mycobacteremia, a longer duration of treatment may be required before a clinical response is observed. Susceptibility testing is not generally recommended as a guide to initial therapy because of the lack of standardization of methods and limited correlation of results with clinical and microbiologic outcome.112,116–118,121,138 Experts recommend testing the susceptibility of MAC isolates to clarithromycin or azithromycin for patients who fail to respond to the initial therapy, who relapse after an initial response, or who develop MAC disease while receiving a macrolide for prophylaxis, although most patients who fail clarithromycin or azithromycin prophylaxis have isolates susceptible to these drugs at the time MAC disease is detected.139,140
Maintenance Therapy (Secondary Prophylaxis)
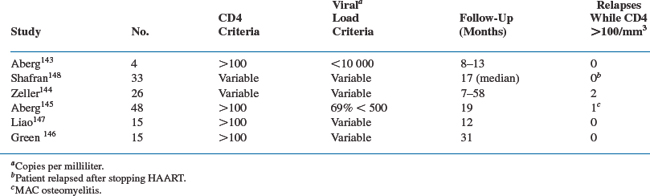
Revised US Public Health Service/Infectious Disease Society of America (USPHS/IDSA) guidelines recommend that therapy for disseminated MAC disease in patients with AIDS be continued for life unless sustained immune recovery occurs with potent antiretroviral therapy.52,138,141,142 The availability of potent combination antiretroviral therapy has altered the outcome of MAC disease in many individuals such that the disease can be effectively ‘cured’ in select patients.143,144 Studies presented to date are summarized in Table 41-2. A nonrandomized prospective study conducted by the ACTG evaluated whether antimycobacterial therapy for disseminated MAC could be withdrawn from subjects who experienced immunologic recovery while receiving potent combination antiretroviral therapy (ART).145 Subjects in this trial had received macrolide-based treatment for their MAC disease for at least 12 months, were asymptomatic for MAC, had received ART for at least 16 weeks, and had CD4+ T-cell counts >100 cells/μL. Forty-eight subjects were enrolled and forty-seven subjects remained MAC free, whereas 1 subject developed localized MAC osteomyelitis. The incidence of MAC infection was 1.44/100 person-years (95% confidence interval, 0.04–8.01) during the median 77 weeks of follow-up. The results of this study support the recommendation that MAC therapy can be safely withdrawn in patients who meet the entry criteria of this trial. Evaluation of small numbers of patients in several more largely observational studies also indicate that patients with established MAC disease who have completed a 12 month or longer course of MAC treatment, remain asymptomatic, and have a sustained increase in the CD4+ T-lymphocyte count to levels higher than 100 cells/μL for 6 months or longer on ART are at low risk for recurrence of MAC disease.143,146,147 Hence the revised USPHS/IDSA guidelines now indicate that discontinuation of chronic maintenance therapy can be safely considered in patients who meet these criteria.52,141–144,148 The guidelines also recommend reinitiation of secondary prophylaxis if the CD4+ T-lymphocyte count falls below 100 cells/μL.
REVIEW OF CLINICAL TRIALS
The early data from human clinical trials upon which the USPHS Task Force recommendations and USPHS/IDSA guidelines for treatment of disseminated M. avium disease are based are captured in a series of what are now largely historical studies. The majority of these were conducted prior to the availability of more potent antiretroviral drugs and combination treatment regimens. The key clinical trials that provide the evidence base for current treatment recommendations are briefly reviewed in the following text and in a summary algorithm presented as Figure 41-1.116,122,124–128,130,149–152
Perhaps the most important study to establish the utility of macrolide-based treatment for M. avium disease in individuals with AIDS is one conducted by the Canadian HIV Trials Network that compared a clarithromycin-containing regimen with a regimen containing conventional antimycobacterial drugs. In this study, 187 patients with AIDS and symptomatic M. avium bacteremia were randomized to receive a combination of rifampin, ethambutol, clofazimine, and ciprofloxacin or clarithromycin (1 g twice daily), ethambutol, and rifabutin (600 mg/day).125 The clarithromycin regimen was associated with a higher proportion of patients who cleared their bacteremia (69% vs 30%; p < 0.001) and with improved survival (8.7 vs 5.2 months; p < 0.001).125 In this study the rifabutin dose was reduced to 300 mg/day after approximately half of the patients assigned to the clarithromycin arm developed uveitis, which was attributed to a previously unrecognized drug interaction between clarithromycin and rifabutin.153,154 A dose–response relationship with rifabutin was suggested by the finding that the 600 mg/day dose was associated with a higher proportion of patients who cleared their mycobacteremia but who developed uveitis than was the 300 mg/day dose.
A series of randomized clinical trials of similar design have compared combinations of two- or three-drug clarithromycin-containing regimens for treatment of MAC disease. In the first of these trials, Chaisson et al evaluated 89 patients with AIDS and M. avium bacteremia who were randomized to receive either clarithromycin and ethambutol or clarithromycin, ethambutol, and clofazimine.130 Although the treatment groups were demographically and clinically comparable, there was a significant difference in baseline quantitative M. avium colony counts between the two arms (152 colony-forming units (CFU)/mL vs 1907 CFU/mL, respectively). The proportion of patients who cleared M. avium bacteremia reported improved symptoms, and relapse rates were similar.130 However, patients randomized to the three-drug arm had a higher rate of treatment-limiting side-effects and a higher mortality rate. In a Cox proportional hazards analysis of factors associated with decreased survival, the baseline quantitative colony counts of M. avium in blood (P = 0.043) and treatment with clofazimine (P = 0.045) were independent predictors of decreased survival.
Dube and coinvestigators evaluated 95 patients with AIDS and MAC bacteremia who were randomized to receive either clarithromycin plus clofazimine or clarithromycin, clofazimine, and ethambutol.128 The primary endpoint was the time to relapse. Although early clinical and microbiologic outcomes were the same for the two treatment arms, 68% of patients in the two-drug arm had relapsed by 36 weeks of therapy compared with only 12% of those on the three-drug regimen (P = 0.004).128 All patients who relapsed had clarithromycin-resistant isolates recovered from blood. Ethambutol appeared to contribute to a reduced likelihood of relapse and emergence of clarithromycin-resistant M. avium in this study.
In a third study, 144 HIV-infected patients were randomized to receive clarithromycin, ethambutol, and rifabutin (450 mg/day) or clarithromycin plus clofazimine; 22 patients randomized to the two-drug arm relapsed compared with only 6 receiving three drugs (p < 0.001).126 Of the 22 patients in the two-drug arm who relapsed, clarithromycin-resistant isolates were recovered from 21, whereas resistant isolates were found in only 2 of 6 in the three-drug arm.126 Both groups had similar clinical responses with reduction in symptoms after 2 and 6 months of treatment, respectively.
Gordin and colleagues compared the combination of clarithromycin plus ethambutol with clarithromycin, ethambutol, and rifabutin (300 mg/day) in 198 patients with AIDS and MAC bacteremia.127 At week 16 a total of 63% and 61% of patients in the three-drug and two-drug arms, respectively, had a bacteriologic response (P = 0.81), with no differences reported in clinical response or median duration of survival. Median survival duration was 439 and 398 days for the three- and two-drug arms, respectively. More-over, no overall differences were noted with regard to the development of clarithromycin resistance; however, when only those with a bacteriologic response were included in the analysis, 2% of those receiving three drugs had a clarithromycin-resistant MAC isolate recovered during therapy compared with 14% of those receiving only clarithromycin plus ethambutol (P = 0.055).127
A fifth study evaluated 203 patients randomized to receive clarithromycin plus either ethambutol, rifabutin, or both drugs.155 The primary endpoint was a complete microbiologic response, defined as two consecutive negative MAC blood cultures at least 1 week apart by week 12 of treatment. The proportion of patients with a complete microbiologic response at week 12 did not differ significantly among the three treatment arms. The proportion of patients with a complete microbiologic response at any time during follow-up were 55%, 46%, and 70%, respectively. The proportion who experienced a relapse on clarithromycin plus rifabutin (24%) was significantly higher than that for the three-drug regimen (6%) (P = 0.027) and marginally significantly higher than for clarithromycin plus ethambutol (7%) (P = 0.057). The rate of emergence of clarithromycin resistance was greater for clarithromycin plus rifabutin than for the other two arms. There was an overall significant difference in time to death (P = 0.020, log-rank test), with improved survival observed in those randomized to the three-drug arm compared to either of the two-drug arms.155
Finally, in a factorial study design attempting to address the potency of higher doses of clarithromycin for treatment of M. avium disease, Cohn et al randomized patients with AIDS and M. avium bacteremia to receive one of two doses of clarithromycin, 500 or 1000 mg twice daily, combined with ethambutol, and they secondarily randomized patients to receive either rifabutin or clofazimine.129 The study was terminated early because of excess mortality observed in the higher-dose clarithromycin arm coupled with the data reported from other clinical trials indicating the lack of benefit of clofazimine and its association with increased mortality. As with the previous studies demonstrating the association of higher doses of clarithromycin with increased early mortality in patients being treated for M. avium disease, the cause was not apparent and the association appeared to be unrelated to adverse events, baseline clinical characteristics, or laboratory abnormalities reported in the study.116,129
Although comparative data are limited, in general, clarithromycin and azithromycin may be used interchangeably.156,157 However, many experts favor the use of clarithromycin due to faster clearing of mycobacteremia and the overall greater clinical trials database demonstrating its efficacy. In the largest published comparative study to date, 246 patients with disseminated MAC disease were randomized to receive azithromycin 250 mg once daily, azithromycin 600 mg once daily, or clarithromycin 500 mg twice daily, each in combination with ethambutol.157 The azithromycin 250 mg arm was halted after an interim analysis showed a lower rate of clearance of MAC bacteremia. At 24 weeks of therapy, the proportion of patients who experienced two consecutive negative MAC blood cultures was similar for both the azithromycin 600 mg qd (n = 68) and clarithromycin (n = 57) arms (46% and 56%, respectively; P = 0.24). The likelihood of relapse, the recovery of resistant isolates following relapse, and mortality were also similar for both treatment arms.157
For patients who fail to respond to initial treatment or who relapse after an initial response, many experts recommend testing M. avium isolates for susceptibility to clarithromycin or azithromycin, and possibly ethambutol and rifabutin, and then using these data to compose a new multidrug regimen consisting of at least two new drugs not previously used and to which the isolate is susceptible. It is unclear whether continuing clarithromycin or azithromycin in the face of resistance provides additional benefit. Preliminary results were reported from one small study in which eight patients who relapsed with a clarithromycin-resistant MAC isolate while receiving a combination of clarithromycin and clofazimine were treated with ‘salvage’ regimens that consisted of continuing a macrolide coupled with ethambutol and one or more additional drugs; five of eight patients had a 1 log10 copies/mL or more decline in mycobacterial colony counts per milliliter of blood, and two of the eight became culture-negative.158 Because the number of drugs with activity against M. avium in this setting is limited, second-line regimens, if constructed in this manner, will likely require two or more of the following:ethambutol, rifabutin, ciprofloxacin, or amikacin with or without continuing a macrolide. Liposomal amikacin could also be considered.159 Based on its lack of efficacy in randomized trials and its association with increased mortality, clofazimine should generally not be used. Other second-line agents, such as ethionamide or thiacetazone, have been anecdotally combined with these drugs, but their role in this setting is unknown.
Animal studies suggest that mefloquine and moxifloxacin have potential for the treatment of macrolide resistant MAC.160 Mefloquine (MFQ), moxifloxacin (MXF), and ethambutol (EMB), in combination, were evaluated against both clarithromycin-resistant (CLR-R) and CLR-susceptible (CLR-S) MAC in a mouse model. After 4 weeks of treatment in mice MFQ was bactericidal, whereas MXF and EMB were bacteriostatic against both CLR-R and CLR-S MAC strains.160 Both the combination of MFQ and EMB and the combination of MFQ and MXF significantly reduced the load of CLR-R MAC in both the liver and the spleen in the mice. Treatment with the three-drug combination resulted in an ∼1 log10 CFU/mL reduction in CLR-R MAC in liver and spleen after 1 week, a 2.1 log10 reduction in CLR-R MAC after 4 weeks, and a 2.17 log10 CFU/mL reduction in MAC in blood. Of note, treatment of a clarithromycin-sensitive strain of MAC (MAC 101) had similar results. Telithromycin, a synthetic erythromycin derivative, has also been shown to be active against MAC in animal studies,161,162 however drug interactions with HIV protease inhibitors may preclude the use of this drug in patients who are taking antiretroviral therapy. None of these approaches has been evaluated in controlled clinical trials in this patient population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree