Chapter 76 Multiple Myeloma and Other Plasma Cell Neoplasms
Epidemiology and Etiology
Radiation has been linked to the pathogenesis of MM, but radiation exposure is found in only approximately 1% of patients. In the Hiroshima and Nagasaki tumor registries, there was no sign of an excess risk of MM.1
Data regarding the role of antigenic stimulation are conflicting. Epidemiologic studies in the United States have demonstrated associations between MM and agricultural workers.2 An increasing trend for the incidence of lymphohematopoietic cancers has been associated with lifetime exposure to the chemical alachlor, a commonly used pesticide. The risk for MM gave a rate ratio of 5.66 in the highest exposure category.3 Other occupational groups associated with the development of MM include miners, workers exposed to wood dust, and sheet metal workers.4
Myeloma plasma cells express several adhesion molecules, including neural cell adhesion molecule (NCAM). Adhesion molecules are involved in homing of plasma cells to bone marrow. Although MM is a neoplasm of end-stage plasma cells, most investigators believe that myeloma stem cells exist as a self-renewing population derived from an earlier compartment. The identity of the cells responsible for the initiation and maintenance of MM remains unclear. Circulating B cells clonally related to MM plasma cells have been reported in some patients with myeloma. Data suggest that myeloma stem cells are CD138− B cells, whereas the terminally differentiated plasma cell is consistently CD138+, though this concept needs further validation. These CD138− B cells can replicate and differentiate into the malignant CD138+ plasma cells.5 Overexpression of BCL-2 and BCL-6 proteins has been seen in clinical myeloma and myeloma cell lines.6 Cytokines, including tumor necrosis factor–alpha (TNF-α), interleukin-1 (IL-1), and IL-6, play an essential role in the biology of the malignancy as well as in mediating the bony manifestations of the disease. Both IL-6 and BCL-2 have been shown to prevent apoptosis, and IL-6 has been implicated as an essential growth factor in MM.7
Pathology and Molecular Genetics
MM is distinct in that the bone marrow is involved in virtually all patients (Fig. 76-1), yet the peripheral blood shows large numbers of circulating cells in only a few patients. Adhesion molecules mediate both homotypical and heterotypical adhesion of tumor cells to either extracellular matrix (ECM) proteins or BM stromal cells. They play a critical role in disease progression. After class switching in the lymph node, adhesion molecules (e.g., CD44, VLA-4, VLA-5, LFA-1, CD56, syndecan-1, and MPC-1) mediate homing of MM cells to the BM stromal cells. Syndecan-1 is a multifunctional regulator of tumor cell growth and survival as well as of bone cell differentiation, and elevated serum syndecan-1 correlates with increased tumor cell mass, decreased metalloproteinase-9 activity, and poor prognosis.8 Syndecan-1 is shed from the surface of most MM cells, induces apoptosis, and can inhibit the growth of MM cells; it also mediates decreased osteoclast and increased osteoblast differentiation.8 Novel agents, including thalidomide, and derivatives (IMiDs), including lenalidomide, as well as the proteasome inhibitor bortezomib9,10 can target both the tumor cell and its BM microenvironment, thereby overcoming cell adhesion-mediated (CAM) conventional drug resistance. TP53 mutations are associated with more advanced myelomas and are related to the terminal phases of the disease.11 Mutations of RAS are more prevalent in MM than in other lymphoid malignant diseases. In a study using genomic DNA in 128 patients, RAS mutations were far more common in patients with aggressive plasma cell leukemias (30%) than in MM patients (9%). The RAS mutations appear to represent a late molecular lesion in the process of myeloma evolution. When levels of RAS were studied in 160 patients with newly diagnosed MM, the median survival rate for patients with mutations of N-RAS was no different from that of patients with no RAS mutations. However, patients with K-RAS mutations had significantly higher tumor burdens at diagnosis and a median survival time of 2 years versus 3.7 years for those who did not have K-RAS mutations. The RAS mutations appear to have an independent impact on the median survival rate of patients with MM.12
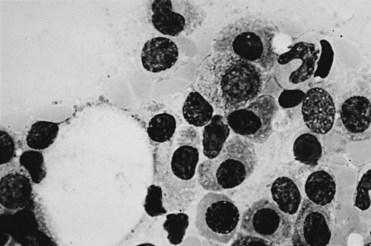
Figure 76-1 Bone marrow diagnostic for multiple myeloma. (Wright stain; original magnification ×1000.)
IgH rearrangements can be found in 75% of patients. Dysregulation of cyclin D1 can be detected in 30% of MM tumors. Cell lines that overexpress cyclin D1 have a translocation detectable into a gamma switch region that suggests an error in VDJ recombination. VH analysis of the clonal cells in MGUS showed much lower mutation frequencies than in MM. The clonogenic cell in MM likely originates from a preswitched but somatically mutated B cell. Genetic studies have demonstrated that the progression of MM from plateau phase to relapse does not involve a new B-cell clone, and progression beyond the plateau phase is not due to clonal succession.13 Advances using molecular probes for FISH have demonstrated aneuploid chromosomes where conventional cytogenetics are normal. Chromosome 13 abnormalities on metaphase cytogenetics have been associated with an unfavorable prognosis in patients with myeloma. The translocation t(11;14) results in up-regulation of cyclin D1 and is the most common translocation detected in patients with MM. Sixteen percent of patients carry the t(11;14) and have better survival and response to therapy rates.14 Immunoglobulin heavy-chain translocations are seen in 60% of patients, and these translocations are more likely to be nonhyperdiploid. Patients with light-chain myeloma never display a functional immunoglobulin heavy-chain recombination. Most patients with light-chain myeloma have one immunoglobulin heavy-chain allele with a germline configuration. The second allele is usually involved in an illegitimate recombination. Light-chain myeloma may be due to the absence of legitimate immunoglobulin heavy-chain rearrangement at the DNA level.15
Clinical Manifestations and Patient Evaluation
Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance
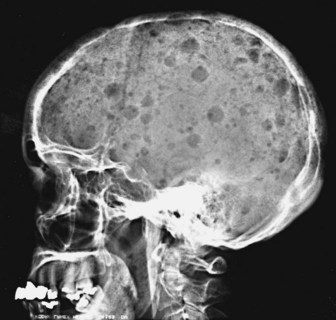
The most common problem is dealing with patients who present with bone pain. Radiographs of the spine in patients with MM frequently show osteoporosis and compression fractures. It is virtually impossible to distinguish the compression fractures associated with MM from those seen in patients with senile osteoporosis (Fig. 76-2). Spine radiographs poorly demonstrate the small lytic lesions frequently responsible for collapse of these vertebrae. All patients with back or rib pain, even with no malignant features on radiographs, should have electrophoresis of serum and urine. If a monoclonal protein is found, radiographs of the entire skeleton often demonstrate lytic lesions in the calvaria, pelvis, and long bones of the humerus and femur (Fig. 76-3).
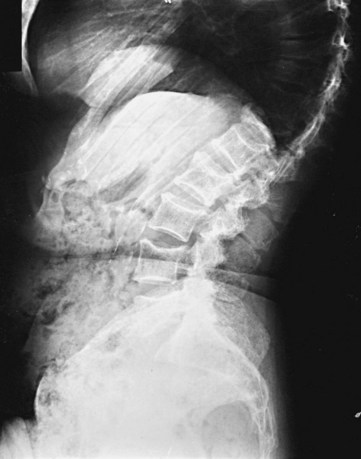
Figure 76-2 Advanced compression fractures of multiple myeloma. Note the lack of features specific for malignancy.
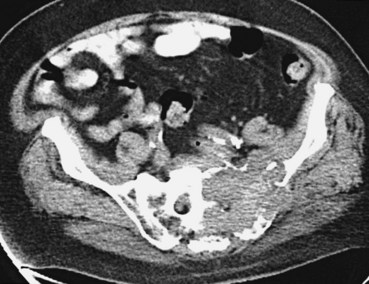
Assessment of bone disease initially requires a radiographic bone survey. Because the lesions of MM are primarily lytic, with little evidence of bony repair, radionuclide bone scans tend to be an inferior approach. In difficult cases in which osteoporosis and monoclonal gammopathy are found with no other changes, computed tomography (CT) scan or magnetic resonance imaging (MRI) of the spine and pelvis can be valuable in detecting clear-cut evidence of neoplasia (Fig. 76-4). Positron emission tomography (PET) may be useful in MM because the lytic lesions are PET avid. When 66 patients were studied with PET and compared with CT and MRI, negative PET findings reliably predicted stable MGUS.16 All patients with active myeloma had focal or diffusely positive scans, four of whom had negative full radiographic bone surveys. Extramedullary uptake was detected by PET in 23% of relapsing patients. PET also tracks response, showing a decline in lesion metabolic activity with successful intervention. It is more sensitive than other imaging techniques, and it can find additional lesions in a third of patients, which affected therapeutic decision making for a quarter of these patients.17
A second clinical problem is distinguishing MGUS from overt MM. In MGUS, the patient is expected to be asymptomatic, without evidence of anemia, hypercalcemia, or renal insufficiency. The patient should have no complaints of rib or back pain. In the absence of symptoms and with a low monoclonal protein value, regular monitoring of the level of the monoclonal protein should suffice. Patients with monoclonal gammopathies, however, should be monitored indefinitely, because the risk for transformation to a malignant plasma-proliferative process is approximately 1% per year. The risk for transformation is predicted by the initial size of the M protein peak. Patients with low monoclonal protein levels (≤0.5 g/dL) had a 6% risk for developing MM at 10 years compared with an 11% risk at 10 years for those with a 1.5-g/dL peak and a 24% risk at 10 years for those with a 2.5-g/dL peak.18
Staging of Multiple Myeloma
The most widely accepted is the International Myeloma Working Group staging system, where stage III myeloma is a β2-microglobulin level higher than 5.5 µg/mL. Stage I includes patients with a β2-microglobulin level that is less than 3.5 µg/mL and a serum albumin level that is less than 3.5 g/dL, and stage II includes all patients who do not fit stage I or stage III (Table 76-1).
TABLE 76-1 International Myeloma Working Group Staging System
| Stage | Criteria |
|---|---|
| I | β2-microglobulin <3.5 µg/mL and albumin ≥3.5 g/dL |
| II | β2-microglobulin <3. µg/mL and albumin <3.5 g/dL or β2-microglobulin = 3.5-5.5 µg/mL |
| III | β2-microglobulin ≥5.5 µg/mL |
The older staging system in clinical use is that of Durie and Salmon (Table 76-2). One problem with the Durie-Salmon staging system is that the criteria for stage I MM in this scheme are also consistent with smoldering MM and indolent MM, which do not require any form of therapy. In most clinical therapy studies, no more than 10% of patients have stage I disease, and many studies exclude patients with stage I from participation. The second difficulty is the subjective nature of interpretation of advanced lytic lesions to distinguish stage II from stage III. No specific well-defined criteria exist to ensure that all institutions use the same definition of advanced MM.
TABLE 76-2 Durie-Salmon Staging System of Multiple Myeloma
| Stage | Criteria |
|---|---|
| I | All of these required: hemoglobin >10 g/dL, Ca2+ <10.5 mg/dL, IgG <5 g/dL or IgA <3 g/dL and light-chain loss <4 g/dL |
| No lytic bone lesions | |
| II | Not fitting stage I or III |
| III | Any one of the following: hemoglobin <8.5 g/dL, Ca2+ >12 mg/dL, IgG >7 g/dL, IgA >5 g/dL, or light-chain loss >12 g/dL |
| Advanced lytic lesions | |
| IIIA | Creatinine <2 mg/dL |
| IIIB | Creatinine ≥2 mg/dL |
Response is generally assessed by the reduction in monoclonal protein; a reduction of 25% to 50% is considered a minor response and 50% to 99% an objective response. Complete eradication of the monoclonal protein in the serum and in the urine with less than 5% plasma cells in the bone marrow is considered a complete response. Patients who either did not have a bone marrow test performed or whose monoclonal protein was no longer visible but detectable by immunofixation are considered to have a near-complete response. In recipients of autologous transplants, a complete response predicts improved outcome after transplantation.19
Primary Therapy for Multiple Myeloma
Patients undergoing therapy for MM should have clinical and laboratory assessment to ensure both safety and efficacy of treatment (Table 76-3). Before each course of treatment, a complete blood count, including differential and platelets, should be done. Serum chemistries should be measured at least every 3 months or more often if clinically indicated. Concomitantly, monoclonal protein in the serum should be measured by immunoelectrophoresis or, preferably, using more sensitive immunofixation techniques and a serum free light-chain assay must be done in patients with light-chain disease. A skeletal survey should be done annually, with BM examination reserved for diagnosis and time of subsequent change in clinical status, in monoclonal Ig, or in hemogram. A bone marrow examination is typically done at diagnosis and subsequently at the time of change in clinical status (i.e., for assessment of response or at time of relapse).
TABLE 76-3 Required Testing to Evaluate Multiple Myeloma
| Category | Test |
|---|---|
| Blood | Complete blood cell count |
| Creatinine | |
| Calcium | |
| Sodium, potassium | |
| Uric acid |