Multinodular Goiter: Pathogenesis and Management
Hans Graf
Introduction
Multinodular goiter (MNG) and diffuse goiter are defined as the enlargement of the thyroid gland, in the absence of autoimmune thyroid disease, malignancy, or inflammation. MNG is associated with more than one nodule identified clinically or surgically. In contrast, a “diffuse” goiter is said to occur when the thyroid gland is enlarged, but there are either no or only scattered subcentimeter nodules within the gland, typically detected only by ultrasound (US). This discussion will focus primarily on MNG. Thyroid function is normal in MNG, but subclinical or overt hyperthyroidism can evolve due to autonomously hyperfunctioning nodules – a clinical entity that is termed “toxic” MNG. Both genetic and environmental factors are involved in the goitrogenic process, but iodine deficiency is the most important risk factor.
The clinical presentation of a patient with MNG can vary from a completely asymptomatic goiter to a life-threatening disease due to upper airway compression. The diagnostic evaluation is based on thyroid function tests, US and cross-sectional imaging, pulmonary function tests, and cytologic analysis. The management of a patient with MNG is guided by the clinical presentation, findings of the diagnostic work-up, the patient’s preferences, and accessibility to therapy.
Epidemiology
By definition, nodular goiter occurs endemically when the goiter prevalence in children from 6 to 12 years old is more than 5%, or sporadically, when this number is less than 5%. It is more prevalent among older individuals and women, especially those living in iodine-deficient areas. Epidemiologic studies of MNG are hampered by limitations such as the method employed for the determination of thyroid volume (palpation, ultrasound, scintigraphy), selection criteria, environmental factors, and thyroid function (1). US is a very accurate method, and studies employing this method have shown that the frequency of thyroid nodular disease (with or without increased thyroid volume) can be as high as 30% to 40% in women and 20% to 30% in adult men living in iodine-deficient areas, with approximately half of that prevalence in younger individuals living in areas with milder iodine deficiency (2). It is estimated that the prevalence of thyroid nodular disease (with increased thyroid volume) is approximately 4% in iodine-sufficient countries (3); this prevalence can be four- to fivefold higher in iodine-deficient areas. In a study evaluating 419 children and 992 adults from an iodine-deficient village of southern Italy, nodular goiter was found in 0.5% of children and 17.0% of adults, by US (4).
In the Wickham study, a population survey of 2,749 persons living in an iodine-sufficient area in Northern England, the prevalence of small goiters (palpable but not visible) was 8.6%, whereas palpable and/or visible goiters were identified in 6.9%. Goiters were four times more common in females (5).
US screening of 635 adults (medium age 56.7 years) without previous history of thyroid disease in Germany (considered as a country with iodine-insufficiency) showed a prevalence of 17% for goiter and of 68% for thyroid nodules, with 25% of women and 40% of men presenting nodular goiter (6). Screening of 101 middle-aged healthy Finnish women identified 3 cases of toxic MNG (7).
In the Framingham Study, conducted in an iodine-sufficient area (Massachusetts in the United States), the prevalence of MNG was 1% by palpation. By US, 3% of men older than 60 years and 36% of women aged 49 to 58 years had thyroid nodules (8). In a prospective study involving 1,544 patients in a general practice in Connecticut, the prevalence of MNG was 0.84% (1.6% in females and 0.1% in males) (9). In a recent review on the prevalence and incidence of endocrine disorders in the United States, no recent data could be found on the prevalence of benign nodular goiter (10).
Natural History
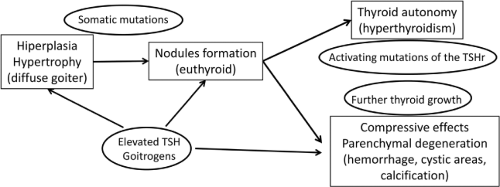
The natural history of MNG is characterized by thyroid growth, followed by nodule formation, and the progression, in many cases, to nodule autonomy. Younger patients generally have small goiters with few nodules, and normal thyroid function. Nontoxic MNG is defined when TSH (thyroid-stimulating hormone) and thyroid hormone levels are within normal range. As the disease progresses, thyroid volume and nodularity increase. Approximately 22% of patients with MNG develop subclinical hyperthyroidism (11), characterized by low TSH and normal free T4. In more advanced stages, overt hyperthyroidism develops (low TSH and high free T4 and/or T3 levels), called toxic MNG. Hyperthyroidism develops as a consequence of the over-production of thyroid hormones due to nodular autonomy, independent of TSH. This autonomy is more frequent in iodine-deficient areas, and is caused by the presence of activating TSH receptor mutations that occur after years of thyroid hyperplasia, and that can be identified even in cases of nontoxic MNG (12,13).
Etiology
The pathogenesis of nontoxic diffuse and nodular goiter is determined by exogenous and endogenous factors. Worldwide, iodine deficiency is the most important factor that increases the
risk for the development of endemic and sporadic goiter, and their prevalence are inversely proportional to the iodine intake (14). According to the World Health Organization, in 2007, nearly 2 billion individuals had insufficient iodine intake (15). In adults, the recommended intake of iodine ranges between 150 and 200 μg/day, with lower values for younger children and higher values for pregnant and lactating women (16).
risk for the development of endemic and sporadic goiter, and their prevalence are inversely proportional to the iodine intake (14). According to the World Health Organization, in 2007, nearly 2 billion individuals had insufficient iodine intake (15). In adults, the recommended intake of iodine ranges between 150 and 200 μg/day, with lower values for younger children and higher values for pregnant and lactating women (16).
There is a direct correlation between the degree of iodine deficiency and the prevalence and size of the goiter, which is a physiologic adaptation to the lack of iodine. A decrease in blood iodine levels leads to a decrease in thyroxine levels (T4), which in turn stimulates the secretion of TSH, in an effort to enhance iodine uptake and restore T4 blood levels. In turn, TSH also stimulates thyroid follicular cell hyperplasia and hypertrophy. In the initial stages, the thyroid parenchyma is diffusely and homogeneously enlarged, but over time nodules often develop. Subsequently, thyroid nodules may become autonomous and secrete thyroid hormones independently of TSH due to activating mutations of the thyrotropin receptor. On the other hand, iodine excess, observed in many countries such as Brazil, Chile, Algeria, Ivory Coast, Zimbabwe, and Uganda, also has a goitrogenic effect due to its action in decreasing the synthesis and secretion of thyroid hormones (17) (see Chapters 11D and 11E).
Several other substances have been known to have goitrogenic effects, such as (i) thiocyanate from cigarettes, (ii) perchlorate from fertilizers, solid propellants for rockets and missiles, fireworks, road flares, matches, and airbag inflation systems, (iii) disulphides from coal processes, (iv) flavonoids from soy, (v) glucosinolates from cruciferous vegetables such as cabbage, kale, cauliflower, broccoli, turnips, and rapeseed, (vi) cyanogenic glucosides from cassava, lima beans, linseed, sorghum, and sweet potato, and (vii) drugs such as lithium and aminoglutethimide (18). Some other environmental pollutants, such as nitrates, are also involved in goitrogenesis (19) (see Chapter 57). The effects of those substances are attributed to their effects on inhibiting iodine uptake and thyroperoxidase activity, on displacing T4 from the serum thyroid-binding protein transthyretin, and on reducing T4 half-life. Those changes lead to a state of hypothyroidism and to an increase in TSH, which stimulates thyroid growth. Moreover, deficiency of selenium, iron, and vitamin A may also predispose to the development of goiter, by (i) increasing peroxides that damage the thyroid gland and impairing deiodinase activity (selenium deficiency), (ii) reducing thyroperoxidase activity (iron deficiency), and (iii) decreasing vitamin A-mediated suppression of the pituitary TSHβ gene (15). The pathogenesis of MNG has also been associated with alterations in the expression of proteins involved in thyrocyte proliferation and in the regulation of TSH receptor, such as Fas and DREAM (downstream regulatory element antagonist modulator), respectively (20,21).
The 2- to 10-fold higher prevalence of goiter in women leads to the assumption that female sex hormones have a direct effect on the pathogenesis of goiter (22). Estrogens stimulate normal and thyroid cancer cell growth (23). However, more recent studies have shown that estrogens can have opposite effects on thyroid cancer cell growth, depending on the balance between ERα and ERβ (24). Moreover, in premenopausal women, estrogen therapy for 1 year did not affect thyroid volume (25), and the use of oral contraceptives seemed to be associated with a decreased risk of goiter (22). The effects of estrogens on the pathogenesis of goiter seem to be determined by several complex pathways, depending on whether endogenous or exogenous estrogens are involved.
TSH is the most important growth factor involved in the pathogenesis of nodular goiter. However, most patients with goiter have normal levels of TSH, and TSH suppression by the administration of levothyroxine may not stop thyroid growth. Therefore, other growth factors are also implicated in the disease. Insulin-like growth factor-1 (IGF-1) levels are positively correlated with thyroid volume in both genders, and with the presence of nodules in men (26,27) (see Chapters 11D and E). Increased levels of IGF-1, as seen in acromegalic patients (28) and in transgenic mice overexpressing IGF-1/IGF-1 receptor (29), are also associated with increased thyroid volume. Not only IGF-1 but also epidermal growth factor (EGF) and fibroblast growth factor (FGF) stimulate proliferation of thyroid follicular cells in vitro and in vivo (30), and the expression of several growth factors is increased in thyroid hyperplasia and nodular goiters (31).
Other growth factors, such as transforming growth factor (TGF), impair thyroid growth by inhibiting thyroid cell growth, increasing apoptosis and blocking TSH-stimulated cAMP accumulation (32). Patients with nodular goiter may have decreased production of TGF, but may be resistant to it, explaining the potential role of TGF in the pathogenesis of goiter (33).
Genetic factors also play a significant role in the pathogenesis of nodular goiter (34) as shown by a study that evaluated twins, and demonstrated that genetic factors accounted for 71% (61% to 78%) of the individual differences in thyroid volume (35). The occurrence of familial cases of nodular goiter, at early ages in many cases, strengthens the hypothesis that genetic factors are involved in the pathogenesis of goiter (36).
Because of their important role in thyroid physiology and hormone synthesis, genes involved in various aspects of thyroid physiology such as TG (thyroglobulin), TPO (thyroperoxidase), SCL5A5 (solute carrier family 5, member 5), SLC26A4 (solute carrier family 26, member 4), dual oxidase 2 (DUOX2), and TSH receptor (TSHR) (36) are major candidate genes for familial euthyroid goiters. However, in familial goiter, most cases have an autosomal-dominant pattern of inheritance. Besides the aforementioned genes, other candidate loci as determinants of nodular goiter are the multinodular goiter 1 (MNG1) locus on chromosome 14, and loci on chromosomes Xp22 (MNG2), 2q, 3p, 3q, 7q, and 8p (31,37,38,39). In genome-wide association studies, four genetic loci were associated with thyroid volume: Two independent loci located upstream of and within capping protein, beta (CAPZB), one within fibroblast growth factor 7 (FGF7), and one on chromosome 16q23 (39,40). Figure 48.1 illustrates the natural history of MNG, and Table 48.1 summarizes examples of situations predisposing to goitrogenesis.
Table 48.1 Examples of Situations Predisposing to Goitrogenesis | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Clinical Manifestations
The clinical manifestations observed in patients with MNG are heterogeneous. Patients with relatively small goiters and with normal thyroid function are usually asymptomatic. On the other hand, patients with large goiters may develop compressive symptoms, such as dyspnea, dysphagia, neck pain or pressure, and hoarseness. Clinical manifestations are influenced not only by the size of the goiter but also by its substernal extension,
since compression of the trachea, esophagus, and great vessels is more likely to occur in the confined space of the thoracic inlet.
since compression of the trachea, esophagus, and great vessels is more likely to occur in the confined space of the thoracic inlet.
There is not a definitive consensus regarding the classification of goiters that extend into and beyond the retrosternal, substernal, intrathoracic, or mediastinal regions (41,42). The most widely used definition of substernal goiter is the one wherein >50% of the total bulk of the thyroid gland extends into the mediastinum. Others consider a substernal goiter as one that descends below the plane of the thoracic inlet. Due to this lack of consensus, the prevalence of intrathoracic goiters, with substernal or mediastinal extension, ranges between 2.6% and 30.4% (43). Goiters that have intrathoracic extension usually lead to the development of earlier and more severe symptoms. Most frequently, the intrathoracic extension occurs into the anterior mediastinum, but about 10% of substernal goiters can be located in the posterior mediastinum (44).
Thyrotoxicosis (i.e., “a clinical state that results from inappropriately high thyroid hormone action in tissues generally due to inappropriately high tissue thyroid hormone levels”) may develop (45), particularly in older patients, and can present from asymptomatic subclinical hyperthyroidism to overt hyperthyroidism. Pain or tenderness are rare manifestations, and occur in the presence of recent intrathyroidal bleeding.
The close relationship of the thyroid gland with adjacent structures is the key factor that determines the most significant clinical manifestations. The symptoms of tracheal compression are dyspnea, wheezing, stridor, cough, and a choking or pressure sensation. These symptoms vary in intensity and may be triggered by exercise or be present even at rest, depending on the gland volume and degree of tracheal compression. A maximal tracheal diameter of <8–10 mm can cause exertional dyspnea, and when the diameter is <5 mm, dyspnea at rest and stridor occur (46).
Goiters with intrathoracic extension may have their symptoms exacerbated during the night when the patient is supine or
lies on one side or the other, or by certain positions that increase tracheal compression. Similarly, acute airway compression may occur (47) particularly if there is a sudden increase in thyroid volume due to thyroid hemorrhage (48) or upper respiratory tract infections contributing to a decrease in tracheal lumen. Compression of the esophagus from retrotracheal growth of a goiter may cause dysphagia. However, this manifestation is less common than the respiratory complications due to the posterior localization of the esophagus. Odynophagia is not a manifestation of thyroidal compression, and is more likely related to inflammatory processes within the pharynx or the esophagus, or acute/subacute thyroiditis.
lies on one side or the other, or by certain positions that increase tracheal compression. Similarly, acute airway compression may occur (47) particularly if there is a sudden increase in thyroid volume due to thyroid hemorrhage (48) or upper respiratory tract infections contributing to a decrease in tracheal lumen. Compression of the esophagus from retrotracheal growth of a goiter may cause dysphagia. However, this manifestation is less common than the respiratory complications due to the posterior localization of the esophagus. Odynophagia is not a manifestation of thyroidal compression, and is more likely related to inflammatory processes within the pharynx or the esophagus, or acute/subacute thyroiditis.
The close relationship of the thyroid gland with the recurrent laryngeal nerves may lead to unilateral or bilateral vocal cord paralysis, which can be transient or permanent. The clinical presentation of recurrent laryngeal nerve compression is dyspnea and hoarseness (49). Similarly, compression of the cervical sympathetic chain may cause paralysis of the phrenic nerve (which can be asymptomatic or present with dyspnea) (50) or Horner’s syndrome (ptosis, miosis, and decreased sweating of the face on the same side) (51). Paralysis of the phrenic nerve and Horner’s syndrome are rare manifestations of goiter and few reports can be found in the literature (52). Thrombosis of the jugular vein and superior vena cava syndrome have also been described (53).
Diagnostic Evaluation
History and Physical Examination
The diagnostic evaluation of a patient with MNG starts with a detailed history and physical examination. Since malignancy is one of the major concerns, signs that raise suspicion must be investigated, such as age (younger than 20 or older than 60 years), male gender, history of radiation to the neck, rapid growth, recent changes in voice, breathing or swallowing, and a family history of thyroid cancer or multiple endocrine neoplasia type 2 (54). Investigating the family history, comorbidities and potential exposure to goitrogens may help to determine etiologic factors.
On physical examination, smaller goiters may become visible only when the patient is asked to swallow or drink a glass of water. The gland must be thoroughly palpated with the physician positioned in front or behind the patient; size, extension, nodularity, and consistency must be estimated by palpation. Younger patients tend to have smaller goiters, with few or no nodules, and without intrathoracic extension. Malignant nodules tend to be firm, irregular and fixed to adjacent tissues. Regional lymphadenopathy also raises suspicion for malignancy. Carotid compression may cause carotid bruit, a systolic sound heard over the carotid artery area during auscultation. Vocal cord paralysis can be roughly evaluated by hearing the patient’s speech. Very large goiters that are primarily substernal may not be palpable, but may cause tracheal deviation that can be detected easily.
Pain is rarely triggered by palpation. Goiters with intrathoracic extension cannot be fully evaluated by palpation. Pemberton sign is pathognomonic of goiters with intrathoracic extension. Pemberton’s maneuver – raising of the patient’s both arms above the head for approximately one minute – increases pressure in the thoracic inlet and in the jugular venous system, causing facial flushing, distended superficial veins of the neck and head, and sometimes inspiratory stridor. This phenomenon has been termed the “thyroid cork.” Symptoms and signs of thyroid dysfunction must also be assessed by medical history and physical examination. Table 48.2 summarizes the possible clinical manifestations seen in patients with MNG.
Table 48.2 Possible Clinical Manifestation in Patients with Multinodular Goiter, and Their Causes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Laboratory Investigation
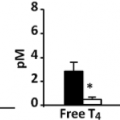
The initial laboratory investigation of a patient with MNG is based on the measurement of serum thyrotropin (TSH), which allows an initial determination of thyroid function. Patients with normal serum TSH are euthyroid and do not need further laboratory investigation (45). Low serum TSH levels are consistent with thyroid hyperfunction, and must be followed by measurements of serum free T4 in order to diagnose subclinical or overt hyperthyroidism. If hyperthyroidism is strongly suspected clinically, serum free T4 can be measured concomitantly with TSH. Measurement of serum triiodothyronine (T3) or free T3 is useful in ruling out T3 thyrotoxicosis in a patient who appears to have subclinical hyperthyroidism. High levels of serum TSH indicate, in most cases, hypothyroidism associated with thyroid enlargement due to goitrous chronic autoimmune (Hashimoto’s thyroiditis). Calcitonin or thyroglobulin measurements are not recommended in the evaluation of malignancy (54), and if measured, calcitonin levels must be interpreted with caution since it has low positive predictive value and can lead to unnecessary surgery (55). In patients with MNG and overt hyperthyroidism, the determination of the titers of antiTSH receptor antibodies may be considered to support or exclude the diagnosis of Graves’ disease co-existing with multiple nonfunctioning thyroid nodules (the so-called Lenhart–Marine Syndrome). Also, elevated antithyroid peroxidase antibodies (TPO Ab) are associated with an increased risk of post-radioiodine hypothyroidism (56,57) and of Graves hyperthyroidism (58).
Imaging
Thyroid US is mandatory in the evaluation of a patient with MNG (54). It is an inexpensive, easy to perform modality, and can be used to guide fine-needle aspiration biopsies (FNAB). It provides an estimate of thyroid volume and also identifies and characterizes benign-appearing and more suspicious thyroid nodules (see Chapter 49). When performing an US, longitudinal and transverse sections of the thyroid gland, as well as areas adjacent to the carotid artery and the jugular vein must be evaluated, in search of suspicious cervical lymphadenopathy.
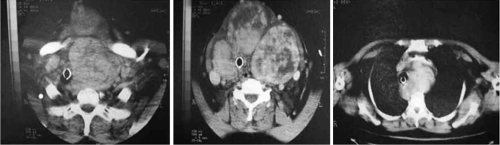
However, the usefulness of US is very limited in patients with intrathoracic goiters, as the US beam cannot penetrate bone. Cross sectional imaging with computerized tomography (CT) or magnetic resonance imaging (MRI) are invaluable tools that fully characterize thyroid volume, degree of substernal extension, and compression of the trachea. Sometimes, both neck and chest CT are needed to fully appreciate the most inferior extent of the goiter. In addition, CT and MRI can determine the cross-sectional area of the trachea, a useful measure of tracheal compression. The main disadvantages of these imaging tools are the costs, the exposure to radiation associated with CT scanning, and the lack of ability to distinguish thyroid nodules that are suspicious for malignancy. When performing CT scanning, iodinated contrast agents should not be used (and are unnecessary) as they will impair future diagnostic or therapeutic use of radioiodine, and can also potentially induce thyrotoxicosis in patients with nontoxic MNG. CT scan is the pre-operative gold-standard for intrathoracic goiters, and can be used to categorize goiters into 3 grades of retrosternal extension: Above the aortic arch, between the aortic arch and the pericardium, and below the right atrium (59). Figure 48.2 illustrates a case of substernal goiter adapted from (47).
Thyroid scintigraphy and radioiodine uptake measurements are not recommended as initial diagnostic tools, but they are necessary for the differential diagnosis of hyperthyroidism in the presence of nodularity (45), to document the intrathoracic extension of large goiters, or to confirm that intrathoracic masses identified by computerized tomography or magnetic resonance are of thyroid origin. Furthermore, the determination of the radioiodine uptake is useful when radioiodine therapy is considered for treatment. In that case, goiters with low and heterogeneous uptake are less responsive to radioiodine therapy, and require higher activities of the radioisotope. When necessary, thyroid scanning is best performed using either technetium 99mTc pertechnetate or 123I. Thyroid scintigraphy is not useful for distinguishing benign from malignant nodules, despite the fact that hot nodules are most frequently benign. The use of thyroid scintigraphy and uptake studies was questioned in a recent study that showed that those tests do not influence diagnosis or treatment outcomes in most cases of hyperthyroidism (60).
Roentgenograms, although inexpensive, are not sensitive techniques. Chest and barium-contrast esophageal x-rays may be useful for documenting tracheal and esophageal compression, respectively. Table 48.3 lists the advantages and disadvantages of the imaging procedures that can be used for the investigation of MNG [adapted from (61)].
Table 48.3 Advantages and Disadvantages of the Main Imaging Tools | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pulmonary Function Tests
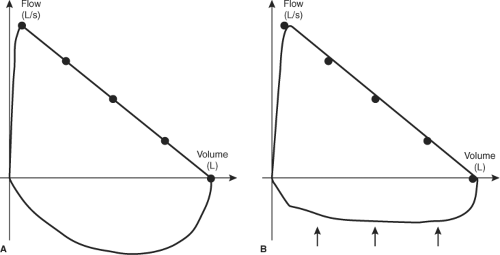
Although large MNGs can lead to tracheal compression and pulmonary function impairment, this problem is probably overlooked by many clinicians (62). This evaluation is fundamental in cases of large and/or intrathoracic goiters compressing the trachea, in order to determine the presence and degree of pulmonary functional impairment, and to demonstrate the efficacy of therapeutic approaches such as surgery and radioiodine therapy. Pulmonary function studies must include flow-volume loop tracings, which show a reduction of inspiratory capacity. Figure 48.3 illustrates flow-volume loops in normal and in MNG patients. Even asymptomatic patients may have abnormal results, and therefore may benefit from this evaluation (62,63). Improvements in pulmonary function after surgery or radioiodine therapy can be objectively quantified with pulmonary function tests.
Table 48.4 Diagnostic Tools Available for the Management of Multinodular Goiter | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Fine-Needle Aspiration Biopsy
The risk of malignancy in patients with MNG (toxic or nontoxic) is the same as the risk in patients with single nodules,
(approximately 5% to 10%) (54,64). However, some studies show that patients with MNG and low TSH levels have a lower risk of papillary thyroid cancer (65). Nevertheless, the recommendations for FNAB in patients with MNG are the same as those for patients with single nodules: The dominant nodule, as well as any sonographically suspicious nodules, should be biopsied (54,66). It is preferable that FNAB be performed with US guidance, which is cost-effective and increases accuracy (67). Nodules that are suspicious for malignancy are hypoechoic, have irregular margins, do not have a sonolucent halo, and have intranodular vascularity and microcalcifications (68) (see Chapter 49).
(approximately 5% to 10%) (54,64). However, some studies show that patients with MNG and low TSH levels have a lower risk of papillary thyroid cancer (65). Nevertheless, the recommendations for FNAB in patients with MNG are the same as those for patients with single nodules: The dominant nodule, as well as any sonographically suspicious nodules, should be biopsied (54,66). It is preferable that FNAB be performed with US guidance, which is cost-effective and increases accuracy (67). Nodules that are suspicious for malignancy are hypoechoic, have irregular margins, do not have a sonolucent halo, and have intranodular vascularity and microcalcifications (68) (see Chapter 49).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree