MORPHOLOGY OF THE OVARY
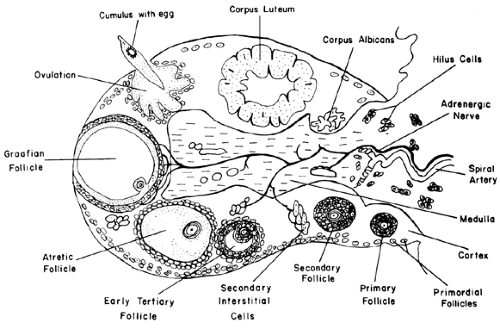
The human ovary is organized into two principal parts: a central zone called the medulla and a predominant peripheral zone called the cortex (Fig. 94-1). The characteristic feature of the cortex is the presence of follicles, containing the female gamete or oocyte, and the corpus luteum. The number and size of the follicles change as a function of the age and reproductive stage of the female. Another feature of the cortex is the presence of clusters of differentiated steroidogenic cells called secondary interstitial cells. They arise from the theca interna of atretic follicles and remain as androgen-producing cells. Characteristically, the medulla contains blood tissue, nerves, and groups of hilus or ovarian Leydig cells (see Fig. 94-1).
FOLLICLES
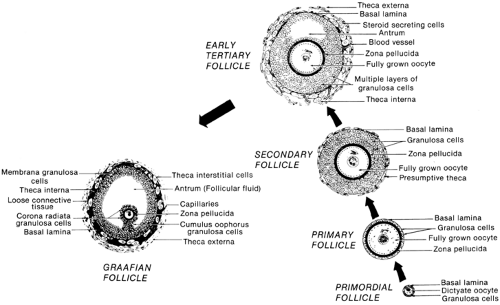
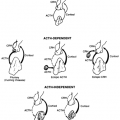
All follicles are located in the cortex, medial to the tunica albuginea, or ovarian capsule. There are two principal classes of follicles: nongrowing and growing. The nongrowing or primordial follicles comprise 90% to 95% of the ovarian follicles throughout the life of the woman. When a primordial follicle is recruited into the pool of growing follicles, its size and position in the cortex change (Fig. 94-2). Typically, the growing follicles are divided into four classes: primary, secondary, tertiary, and graafian (Fig. 94-3; see Fig. 94-2). Intrinsic signals are required for, and are important to, the development of preantral follicles (primary, secondary, early tertiary). Hence, the preantral stages of folliculogenesis are gonadotropin-independent. By contrast, the graafian stages (small, medium, large) are gonadotropin-dependent. The growing follicles that do not participate in ovulation undergo apoptosis (programmed cell death) and become atretic follicles.
PRIMORDIAL FOLLICLE
The ability of a woman to have a menstrual cycle totally depends on having a pool of primordial follicles. Consequently, primordial follicles represent the fundamental reproductive units of the ovary. Histologically, primordial follicles possess a simple organization: a small oocyte arrested in diplotene of the first meiotic prophase, a surrounding layer of follicle cells (i.e., future granulosa cells), and a basal lamina (see Fig. 94-3). Primordial follicles do not have a theca and therefore do not have an independent blood supply.1
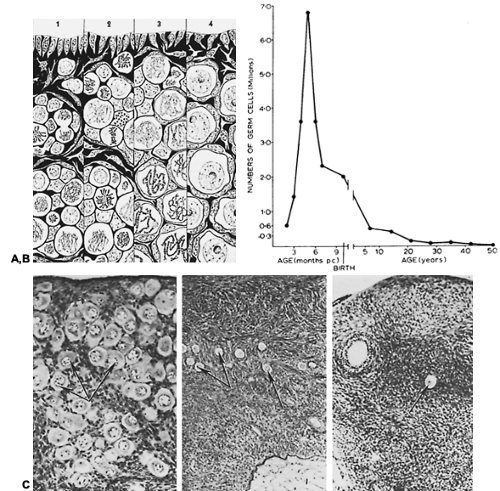
All primordial follicles are formed in the fetal ovaries2 at between 6 and 9 months of gestation (Fig. 94-4). Because each germ cell has entered meiosis, there are no gametes capable of dividing mitotically. All oocytes capable of participating in reproduction during a woman’s life are formed before birth. In human females, recruitment (i.e., the initiation of primordial follicle growth) begins in the fetus and continues until menopause.2 As a result of recruitment, the size of the pool of primordial follicles becomes progressively smaller with age; between birth and menarche, the number of primordial follicles decreases from several million to several hundred thousand (see Fig. 94-4). The number of primordial follicles continues to decline until they are relatively rare at menopause.3
PRIMARY FOLLICLE
A primary follicle contains a growing oocyte surrounded by one layer of granulosa cells (see Fig. 94-2 and Fig. 94-3). The process
of primary follicle formation begins when the squamous granulosa cells round up and appear cuboidal.4 After this occurs, the meiotic chromosomes enter the lampbrush state, and the oocyte begins to increase in size by virtue of increased RNA and protein synthesis.2,5 Small patches of oocyte-derived material appear between granulosa cells. Eventually, this extracellular matrix (i.e., zona pellucida [ZP]) covers the entire oocyte. By the late primary stage, the oocyte, encapsulated by the ZP, is almost full-grown (˜100 μm in diameter). The human ZP is composed of three glycoproteins termed ZP-1, -2, and -3.6 The ZP-3 glycoprotein functions as the primary sperm receptor and induces the acrosome reaction.7 Anti–ZP-3 antibodies can block fertilization, and attempts are under way to utilize ZP-3 as an immunogen to develop a human contraceptive vaccine.8,9
of primary follicle formation begins when the squamous granulosa cells round up and appear cuboidal.4 After this occurs, the meiotic chromosomes enter the lampbrush state, and the oocyte begins to increase in size by virtue of increased RNA and protein synthesis.2,5 Small patches of oocyte-derived material appear between granulosa cells. Eventually, this extracellular matrix (i.e., zona pellucida [ZP]) covers the entire oocyte. By the late primary stage, the oocyte, encapsulated by the ZP, is almost full-grown (˜100 μm in diameter). The human ZP is composed of three glycoproteins termed ZP-1, -2, and -3.6 The ZP-3 glycoprotein functions as the primary sperm receptor and induces the acrosome reaction.7 Anti–ZP-3 antibodies can block fertilization, and attempts are under way to utilize ZP-3 as an immunogen to develop a human contraceptive vaccine.8,9
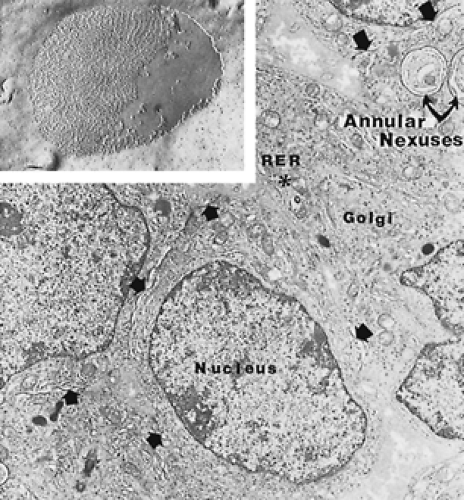
The development of primary follicles leads to an increase in the number and size of gap junctions between the granulosa cells (Fig. 94-5) as well as between the granulosa and the oocyte.10 Gap junctions consist of a family of proteins called connexins (Cx).10,11 In the case of animal follicles, Cx43 is the major gap junction protein between granulosa cells,10 while Cx37 is the major gap junction protein between the oocyte and granulosa cells.12 Cx37 is an oocyte-derived protein; results from studies of Cx37-deficient mice have shown that Cx37 is obligatory for folliculogenesis and fertility.12 The role of Cx37
in human ovary physiology and pathophysiology remains to be determined.
in human ovary physiology and pathophysiology remains to be determined.
SECONDARY FOLLICLE
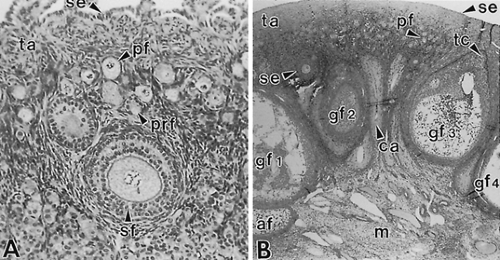
A secondary follicle contains two to eight layers of granulosa cells with no antrum. During secondary follicle development, the granulosa cells proliferate slowly,4 and the oocyte completes its final growth.13 By the end of the secondary stage, the follicle is a multilayered structure that is strikingly symmetric; in the center is a full-grown oocyte (˜120 μm in diameter), eight layers of stratified low columnar granulosa cells, and a basal lamina (Fig. 94-6). When the follicle has two to three layers of granulosa cells, a signal (yet to be identified) is generated that causes a stream of mesenchymal cells to migrate toward the basal lamina.14 They become organized into a layer of fibroblast-like cells (see Fig. 94-6) that ultimately develops into the theca interna and the theca externa. At about this time, the secondary follicle acquires a set of capillaries. The vessels form two sets of interconnected capillaries, an inner wreath located in the theca interna, which is supplied by branches from an outer wreath located in the theca externa.1 Call-Exner bodies develop among the granulosa cells in the secondary follicle (see Fig. 94-6). Histologically, these bodies appear to be made of extracellular matrix. The physiologic function of Call-Exner bodies is unknown; however, given the importance of the extracellular matrix in proliferation and cytodifferentiation,15 they may play a role in generating subtypes of granulosa cells by providing novel substrate-to-cell interactions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree