MORPHOLOGY AND HORMONAL CONTROL
COMPARATIVE ANATOMY OF LACTATION
The constituents of milk products differ widely among species, undoubtedly reflecting differences in the nutritional requirements of the neonate and the environmental restrictions on the mother. The mammary gland is unique in the animal kingdom in that only 4200 species of mammals possess this organ. Most of these mammals (95%) belong to the subclass Eutheria; the remainder belong either to the subclass Monotremata, which contains the primitive egg-laying mammals such as the duckbill platypus, or to the Metatheria, which contains the single-order Marsupilia (i.e., kangaroos).1
HISTORY OF THE HORMONAL CONTROL OF THE BREAST
Haller, in 1765, was the first to conclude that milk was derived from blood. The relation of blood and milk production was investigated by Sir Astley Cooper, who first described the early physiologic occurrence of milk letdown and lactogenesis. In the 1930s, it was shown by means of pressure monitors that milk secretion and ejection are separate events.2 In 1928, prolactin was extracted and demonstrated to be different from other known pituitary hormones.3 In the 1940s, it was proposed that during pregnancy, estrogen and progesterone promote full mammary growth while progesterone inhibits estrogen stimulation of prolactin secretion, and that at parturition, an increase in circulating prolactin and cortisol accompanied by a fall in estrogen and progesterone trigger lactation.4 Although incorrect in some aspects, this hypothesis endured for more than 20 years. It began to be challenged with the discovery that mammary growth occurs in the absence of steroid hormones in adrenalectomized and gona-dectomized rats that are recipients of pituitary mammotrophic tumor xenografts secreting prolactin, growth hormone, and adrenocorticotropic
hormone.5 Subsequent studies showed that estrogen stimulates the secretion of prolactin.6 Partially inhibiting the response, progesterone suppresses prolactin secretion below baseline. It has been proposed that elevated progesterone levels during pregnancy prevent the secretion of milk and that the withdrawal of this hormone after parturition is in part responsible for lactogenesis.7
hormone.5 Subsequent studies showed that estrogen stimulates the secretion of prolactin.6 Partially inhibiting the response, progesterone suppresses prolactin secretion below baseline. It has been proposed that elevated progesterone levels during pregnancy prevent the secretion of milk and that the withdrawal of this hormone after parturition is in part responsible for lactogenesis.7
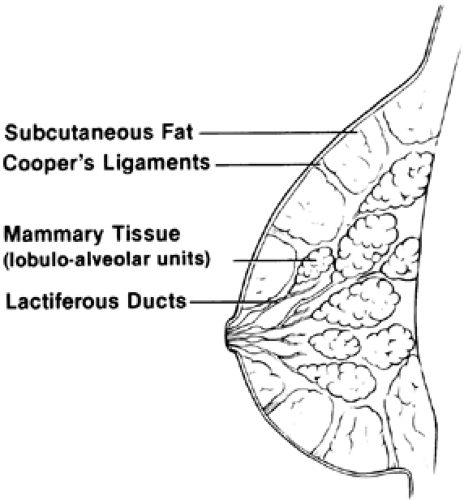
ANATOMY OF THE MAMMARY GLAND
The mammary gland lies on the pectoralis fascia and musculature of the chest wall over the upper anterior rib cage (Fig. 106-1). It is surrounded by a layer of fat and encased in skin. The tissue extends into the axilla, forming the tail of Spence. The mammary gland consists of 12 to 20 glandular lobes or lobules that are connected by a ductal system. The ducts are surrounded by connective and periductal tissues, which are under hormonal control. The lactiferous ducts enlarge as they approach the nipple, which is pigmented and surrounded by the areola. The ductal tissue is lined by epithelial cells. The individual functional unit of the breast is the alveolar cell, which is surrounded by the hormonally responsive myoepithelial cells. Milk is produced at the surface of the alveolar cells and is ejected by the contraction of the myoepithelial cells under the influence of oxytocin. Fibrous septa run from the lobules into the superficial fascia. The suspensory ligaments of Cooper permit mobility of the breast.
The principal blood supply of the breast comes from the lateral thoracic and internal thoracic arteries, although components have been identified from the anterior intercostal vessels. The breast is innervated chiefly by the intercostal nerves carrying both sensory and autonomic fibers. The nipple and areola are innervated by the interior ramus of the fourth intercostal nerve. Seventy-five percent of the lymphatic drainage involves axillary pathways through the pectoral and apical axillary nodes. Drainage also occurs through parasternal routes.
EMBRYOLOGY AND HISTOLOGY OF THE MAMMARY GLAND
The mammary gland can be identified 6 weeks after fertilization; it is derived from ectoderm. At 20 weeks’ gestation, the 16 to 24 primitive lactiferous ducts invade the mesoderm. These ectodermal projections continue to branch and grow deeper into the tissue. Canalization occurs near term. Importantly, although the central lactiferous duct is present at birth, the gland does not differentiate until it receives the appropriate hormonal signals.
By the time an embryo is 7 mm in length, the mammary tissue has thickened to form a ridge (known as the mammary crest or milk line) extending along the ventrolateral body wall from the axillary to the inguinal region on each side. The caudal epithelium regresses, and the crest in the thoracic region thickens further to form a primordial mammary bud by the time the embryo is 10 to 12 mm in length. These embryologic origins account for the occasional development of supernumerary nipples and accessory breast tissue.
Although mammary tissue remains relatively unresponsive until pregnancy, it is responsive to systemic hormone administration during fetal life. In the third trimester, when fetal prolactin levels increase, terminal differentiation of ductal cells occurs. This hormonal milieu accounts for the witch’s milk expressible from the nipples of some normal newborn girls. After birth, these cells revert slowly to a more primitive state.8 The glands remain quiescent until the establishment of ovulatory menstrual cycles, at which time breast development proceeds in the manner described by Marshall and Tanner9 (see Chap. 91). Although the hormonal regulation of mammogenesis is unclear, estrogen in vivo appears to bring about ductal proliferation, although it has little ability to stimulate lobuloalveolar development.10 In vitro, however, estrogens do not promote mammary growth. It has been suggested that various epidermal growth factors participate in this process.11 When progesterone is administered in vivo, lobuloalveolar development occurs.12 However, the administration of estrogen and progesterone to hypophysectomized animals fails to promote mammogenesis.13 These data strongly suggest that hormones other than estrogens and progestogens play a role in mammogenesis. For instance, if the pituitary and adrenal glands are removed from oophorectomized rats, the addition of estrogen plus corticoids and growth hormone restores duct growth similar to that seen in puberty.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree