Grade I:
Events carrying minor risks; complication, if left untreated, resolves spontaneously or at most requires a simple bedside procedure. Analgesics, antipyretics, antiemetics, antibiotics orally, and antidiarrheals are permitted
Grade II:
Potentially life threatening; requires some form of intervention.
Grade IIa
requires drugs other than above, parental nutrition, or transfusions.
Grade IIb
requires invasive procedures (radiological or endoscopic) or reoperation
Grade III:
Events with residual or lasting disability, including organ resection
Grade IV:
Complications result in death
Grade I: | Minor complications; resolves spontaneously if left untreated or requires a simple bedside procedure. Analgesics, antipyretics, antiemetics, antibiotics orally, and antidiarrheals are permitted. Examples include superficial surgical-site infection, urinary retention, lower urinary tract infection, ileus requiring nasogastric tube |
Grade II: | Potentially life threatening; requires intervention that carries some risks. Grade IIa requires other drugs, parental nutrition, or transfusions. Examples include pneumonia, arrhythmia, or acute pancreatitis. Grade IIb requires invasive procedures (radiological or endoscopic) or reoperation. Examples include computed-tomography-guided abscess drainage |
Grade III: | Events with residual or lasting disability or organ loss; includes cerebrovascular events with residual disability and iatrogenic splenectomy. All complications categorized as cardiac, respiratory, gastrointestinal, urinary, local, or other |
Grade IV: | Complications result in death |
Grade 0: | No complication |
Grade I: | Complications requiring either no or minor intervention, such as antibiotics orally, bowel rest, or basic monitoring |
Grade II: | Complications requiring moderate interventions, such medication intravenously (e.g., antibiotics or antiarrhythmics), total parental nutrition, prolonged tube feeding, or chesttube insertion |
Grade III: | Complications requiring hospital readmission, surgical intervention, or radiological intervention |
Grade IV: | Complications producing chronic disability, organ resection, or enteric diversion |
Grade V: | Complications result in death |
Grade I: | No complication |
Grade II: | Minor complications; wound infection, urinary tract infection, pancreatitis, ileus, deep vein thrombosis |
Grade III: | Major complications; requiring reoperation on intensive care admission or interventional radiology treatment |
Grade IV: | In-hospital or intensive care unit mortality |
Hematological disorders | Infections |
Gastrointestinal disorders | Hair disorders |
Renal-bladder disorders | Cardiac disorders |
Pulmonary-allergic disorders | Neurotoxicity disorders |
Cutaneous disorders | Constipation disorders |
Fever, drug disorders | Pain |
14.3 Risk Factors
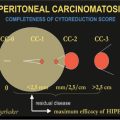
Many risk factors associated with the appearance of adverse events during CRS plus HIPEC are described by many authors. However it is not possible to compare the various series due to the numerous variables used by the different authors. Consequently, we report the most representative series: Kusamura et al. [6], using univariate analysis, found the following variables have a statistically significant correlation with major morbidity: male gender (p = 0.016), Eastern Cooperative Oncology Group (ECOG) performance status (p = 0.05), no previous systemic chemotherapy (p = 0.004), carcinomatosis extent (p = 0.027), number of bowel anastomoses > 2 (p = 0.028), procedure duration (p = 0.014), extent of cytoreduction (p = 0.019), and cisplatin (CDDP) dose for intraperitoneal hyperthermic perfusion > 240 mg (p = 0.02). However, in multivariate analysis, no previous systemic chemotherapy [odds ratio (OR) 2.719; 95% confidence interval (CI) 0.984–7.512; p = 0.054], extent of cytoreduction (OR 2.877; 95% CI 1.292–6.404; p = 0.01), and CDDP dose > 240 mg (OR 3.128; 95% CI 1.239–7.900; p = 0.016) were independent risk factors for major morbidity. Glehen et al. [9] carried out only a univariate analysis in which they found that major morbidity was statistically linked with carcinomatosis stage (p = 0.016), duration of surgery (p = 0.005), and number of resections and peritonectomy procedures (p = 0.042). Hansson et al., [10], in their multivariate analysis, observed the adverse events were associated with stoma formation, duration of surgery, perioperative blood loss, and Peritoneal Cancer Index (PCI). Casado-Adam et al. [11], in their univariate analysis, found a statistically significant correlation between morbidity, histological grade (p = 0.0166), PCI (p = 0.0049), small-bowel resections (p = 0.0493), colorectal anastomosis (p = 0.0430), and the number of anastomoses performed per patient (p = 0.0288). However, multivariate analysis showed that PCI was the only independent risk factor for gastrointestinal complications (p = 0.0586). Finally, Mizumoto et al. [12], in their univariate analysis, showed that PCI > 20, operation time > 5 h, and blood loss > 2.5 L were significant risk factors for postoperative complications. On the other hand, the complication rate in patients who received HIPEC was significantly lower than in patients treated without HIPEC. Gender, age >/< 65 years old, origin of peritoneal carcinomatosis (PC), and completeness of cytoreduction were not related to morbidity. Multivariate analysis showed that PCI higher than 20 was the only significant factor that increased the occurrence of postoperative complications. PCI > 20 was associated with 2.8 times increased risk of postoperative complications. In multivariate analysis, patients who received HIPEC showed significantly lower mortality and morbidity rates than patients who did not.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree