Fig. 14.1
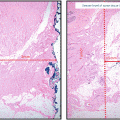
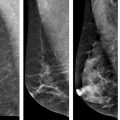
Baseline and follow-up mammograms in a 51-year-old woman with initial diagnosis of DCIS, treated with breast-conserving surgery and adjuvant radiation. Left breast mammogram at initial diagnosis and at recurrence. a Cranio-caudal (CC). b CC magnification view showing cluster of pleomorphic microcalcifications in the left medial breast at diagnosis. c CC. d CC magnification view left breast 10 months following radiation, demonstrating new faint linear microcalcifications in the same quadrant as the index DCIS. Core biopsy demonstrated recurrent DCIS
Although the guideline consensus recommends annual mammographic surveillance, one retrospective review has demonstrated a benefit from semi-annual ipsilateral surveillance for women that have undergone successful BCS [38]. These researchers found that semi-annual surveillance with mammography detected recurrences at an earlier stage when compared to annual surveillance, which may translate to a survival benefit. This study has not changed the current guidelines, but it does suggest that further studies and prospective evaluation are needed to determine the most effective surveillance frequency .
Current data and consensus panels support routine clinical examination and posttreatment annual mammographic surveillance starting 1 year after the initial mammogram and at least 6 months after the completion of radiation treatment.
MRI
Magnetic resonance imaging (MRI) is an increasingly used imaging tool in the evaluation and management of breast cancer. The potential clinical applications of MRI have expanded beyond the concrete evidence that supports its use. Although survival data are not available, annual screening MRI has been accepted for women with known or suspected breast cancer susceptibility gene (BRCA) mutations and women that have an equivalent lifetime risk > 20 % [39, 40]. Overall, MRI is not recommended for routine posttreatment surveillance for breast cancer [33]. However, it would be reasonable to continue MRI surveillance for women who meet the criteria for annual MRI screening and have undergone BCS for DCIS.
MRI is the most sensitive imaging modality for detecting all tumor types (88–100 %) [41–48]. Specifically with respect to DCIS, MRI has been shown to be more accurate than mammography in detection and determining the extent of disease, especially high-grade lesions [47, 48]. MRI has shown promise in detection of occult invasion in DCIS and measuring tumor response to neoadjuvant hormonal therapy [49, 50]. Prospective studies evaluating neoadjuvant hormonal therapy for women with DCIS in which serial MRI is used to monitor the response are ongoing. Although there is much promise for the potential uses of MRI in patients with DCIS, MRI is expensive and has a moderate specificity (37–70 %) [51–55] generating false positives and unnecessary biopsies. Due to these valid concerns, MRI has not been able to surpass mammography’s ability when balanced with cost-efficacy. Survival data is not available for MRI in the detection of breast cancer.
Current data do not support the routine use of MRI after treatment for DCIS. Surveillance MRI should be considered if a patient has a lifetime risk > 20 % of breast cancer.
Positron Emission Tomography/Computed Tomography
Distant metastasis and death after treatment for DCIS are not common; but when it occurs, it is usually simultaneous with an invasive breast cancer recurrence [56]. Based on a systematic review, researchers concluded that the use of positron emission tomography/computed tomography (PET/CT) would not be cost-effective in every breast cancer survivor suspected of having a recurrence [57]. Women treated for DCIS have a much lower likelihood of a distant recurrence than women with a history of invasive disease and would have even less justification for routine PET/CT as part of a surveillance program. If a study were needed to evaluate symptoms suspected to be related to a recurrence, PET/CT demonstrated an advantage over PET alone for the diagnosis of recurrent breast cancer and is currently considered a useful adjunct when combined with conventional imaging [58].
Current data do not support the routine use of PET/CT in surveillance after treatment for DCIS.
Additional Surveillance Studies
Randomized trial and meta-analysis evaluation compared clinical visits and mammography with more intensive follow-up including bone scans, liver ultrasonography, chest radiographs, and laboratory testing among women treated for breast cancer [36, 59, 60]. These studies found no significant disease-free or overall survival advantage using the intensive surveillance regimen. Furthermore, the intensive regimen did not improve the quality of life [59]. Thus, there is no role for such intensive surveillance regimens in early-stage breast cancer, and even less justification for patients with DCIS.
Blood tests, bone scans, chest radiographs, and liver ultrasounds are not recommended for routine surveillance after DCIS in asymptomatic patients with no clinical evidence of disease.
Future Directions and Summary
Active Surveillance for Invasive Disease
Since there is limited ability to predict which women with DCIS will (or will not) progress to invasive disease in the absence of treatment, surgical excision presently remains part of the recommended treatment algorithm. However, there is increased interest in an “active surveillance” option for well-informed women who choose to decline surgery. It is important to follow these high-risk women with close radiographic surveillance because DCIS is often nonpalpable. The goal of such surveillance would be aimed towards early detection of invasive progression, rather than detection of DCIS itself [61]. Although the end points and effectiveness of surveillance in this setting require further study, one option would be to follow these patients similar to known BRCA-mutation carriers, with alternating annual bilateral mammography and annual bilateral breast MRI , since the latter has been shown to have higher sensitivity for invasive cancer than mammography alone [50]. Future research will determine which patients are the best candidates for such an approach, weighing the benefits of treatment against the uncertainty of active surveillance.
Summary
DCIS is a frequently encountered diagnosis among a screened population of women, and its detection may increase with more prevalent use of breast MRI as well as other advanced breast imaging technologies. Surveillance guidelines for women treated with invasive cancer are generally applied for DCIS, although the comparatively low risk of invasive recurrence and negligible likelihood of metastatic disease makes the implications of screening different from those for invasive cancer. Current guidelines call for the routine use of mammography and physical examination after treatment for DCIS, and a summary of recommendations are outlined in Table 14.1, with a different surveillance strategy according to initial treatment for DCIS. For both index and contralateral breasts that remain at risk, annual screening is recommended beyond 5 years from diagnosis. Additional studies will help establish the upper age limit for surveillance as well as the optimal incorporation of emerging technologies in surveillance following DCIS treatment.
Table 14.1
Surveillance guidelines for patients after treatment for DCIS
Patient | Clinical breast examination | Imaging |
|---|---|---|
Mastectomy without reconstruction | Every 3–6 months for 3 years, every 6–12 months for 2 years, then annually | No surveillance imaging required for index breast |
Mastectomy with implant-based reconstruction | Every 3–6 months for 3 years, every 6–12 months for 2 years, then annually | No surveillance imaging required for index breast |
Mastectomy with autologous tissue reconstruction | Every 3–6 months for 3 years, every 6–12 months for 2 years, then annually | No surveillance imaging required for index breast |
Partial mastectomy with or without radiation | Every 3–6 months for 3 years, every 6–12 months for 2 years, then annually | Annual mammography starting 1 year after initial mammogram and at least 6 months after completion of radiation therapy |
No surgical excision | Every 3–6 months for 3 years, every 6–12 months for 2 years, then annually | Baseline MRI at diagnosis, then alternating breast MRI and mammography every 6 months |
References
1.
Brinton LA, Sherman ME, Carreon JD, et al. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643–8.PubMedCentralPubMedCrossRef
2.
3.
4.
5.
6.
Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from national surgical adjuvant breast and bowel project B-17. J Clin Oncol. 1998;16:441–52.PubMed
7.
8.
9.
Schouten van der Velden AP, van Vugt R, Van Dijck JA, et al. Local recurrences after different treatment strategies for ductal carcinoma in situ of the breast: a population-based study in the East Netherlands. Int J Radiat Oncol Biol Phys. 2007;69:703–10.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree