Variable
BRCA1
BRCA2
Surrounding invasive tumor
< Sporadic controls
= Sporadic controls
Presence in prophylactic mastectomy specimens
Increased in women more than 40 years of age
Increased in women over 40 years of age
Prevalence in unselected patients
0.8 %
2.4 %
Prevalence in patients presenting for genetic counseling
10 %
17 %
Grade
High
Similar to sporadic controls
Absolute risk for mutation carriers
Sixfold
Sixfold
Characteristics of DCIS in the Context of Cancer Family Syndromes
Similar to its invasive counterpart, DCIS is a heterogeneous disease across individuals, with distinct molecular pathology and phenotypic outcomes based on mutation carrier status . For example, DCIS associated with invasive disease in BRCA1 carriers tends to show a more basal-like phenotype with low expression of estrogen receptor (ER) , progesterone receptor (PR), and Her2/neu (Her2) while positive for cytokeratin and epidermal growth factor receptor (EGFR). BRCA2 carriers however tend to display a more luminal-type pattern, frequently staining positive for ER/PR and negative for cytokeratin and EGFR. Not surprisingly, DCIS in BRCA1 carriers is more likely to be highly proliferative. In IBC, these molecular subtypes greatly impact the phenotypic outcomes in patients, and this study suggests that these crucial drivers of cancer are already determined in the preinvasive stage [20].
There also exist some similarities between DCIS in BRCA1 and BRCA2 carriers with regard to expression of certain hypoxia markers. Hypoxia-inducible factor-1α (HIF-1α) expression was detected in 63 % of BRCA1 (n = 32) and 62 % of BRCA2 (n = 16) as compared to 34 % of non-BRCA mutation-related (n = 77) DCIS cases (p = 0.005). Similar overexpression of CAIX and Glut-1 was seen in the BRCA-related cases. The expression of these hypoxia-related proteins in BRCA mutation-associated DCIS was similar to the expression in the matched invasive cancer in 60 % or more of cases [21]. These unique biochemical and molecular characteristics of DCIS in BRCA carriers have important implications for biomarkers of early detection and targeted treatment (Table 15.2).
Table 15.2
Immunohistochemical profile of DCIS in BRCA1/2 mutation carriers (n = 28)
Characteristic | BRCA1 | BRCA2 |
|---|---|---|
ER negative | 17 | 1 |
ER positive | 8 | 8 |
PR negative | 32 | 5 |
PR positive | 4 | 4 |
Her2 negative | 25 | 6 |
Her2 positive | 0 | 3 |
DCIS lesions in BRCA1 mutation carriers were:
Mostly basal type
Low ER/PR/Her2
Frequently expressing CK5/6, CK14, and EGFR
Grade 3, with high proliferation
DCIS lesions in BRCA2 mutation carriers were:
Mostly luminal type
Frequent expressions of ER and PR
Infrequent CK5/6, CK14, and EGFR expression
Grade 3, with low proliferation
There exists a variety of other germline mutations associated with hereditary breast cancer syndrome including ATM, PALB2, NBN, BRIP1, MRE11, RAD51C, RAD50, STK11, CDH1, TP53, CHEK2, and PTEN. While their associations with IBC are relatively well defined, there has not been any large study to date focusing strictly on DCIS in persons harboring variants in these genes (Fig. 15.1). A smaller study of 43 malignant breast specimens from 39 women with known TP53 mutations included 32 invasive ductal carcinomas and 11 DCIS cases. The women in this study had an earlier average age of onset of DCIS (34 years), and their DCIS was noted to be of high nuclear grade, with 55 % being ER/PR + and 73 % Her2(+) [22]. Further studies of DCIS in individuals who carry these rarer germline mutations in non-BRCA genes should be pursued as this hereditary population is likely to benefit most from earlier detection and targeted treatment of their malignant lesions .
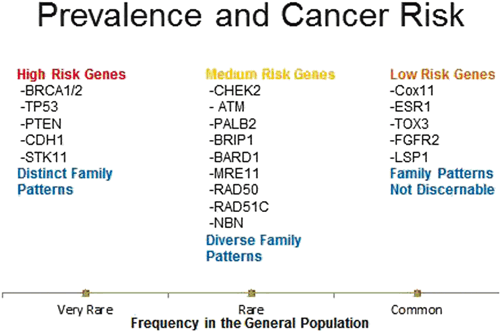
Fig. 15.1
Inherited germline mutations in breast cancer
It is important to note that since it has been only a few months that we have been more commonly testing for germline mutations in these non-BRCA genes, we cannot, at this point in time, speculate as to what percentage of the total burden of DCIS present in cancer families is attributable to any one of these genes. We need to entertain the possibility that, when more individuals are tested, we may find that these mutations are in aggregate responsible for a very significant number of cases of DCIS, perhaps in numbers comparable to the BRCA genes, but possibly at lower risk in each individual person.
Early Detection by Circulating Epithelial Cells and Future Research
Recent studies have highlighted the importance of circulating tumor cells (CTC) as an independent prognostic indicator of progression-free survival and breast cancer-related death. CTCs are found in ~ 70 % of metastatic breast cancer cases, and the presence of CTCs prior to neoadjuvant chemotherapy is associated with a significant risk for breast cancer recurrence. A study of 602 patients undergoing breast surgery showed that having CTCs in peripheral blood prior to curative surgery was associated with an increased risk of breast cancer-related death. Interestingly, they found similar percentage of women with DCIS had CTCs as compared to invasive disease (19 %), though those women with DCIS were excluded from further follow-up analysis or risk outcomes [23]. This study raises an interesting thought that CTCs may set the milieu for metastasis even as early as the preinvasive stage.
A vital clinical question that remains largely unanswered is how to better determine which cases of DCIS will recur and which will go on to develop invasive disease. Molecular subtyping analysis has identified differences at the protein level of DCIS specimens from individuals who subsequently go on to develop invasive cancer versus those who have recurrence of DCIS alone. Interestingly, in this study, a family history of breast cancer was not associated with disease recurrence or progression to invasive disease [24]. Clinical prediction tools have been proposed to better predict DCIS recurrence. For example, Memorial Sloan Kettering Cancer Center’s Breast Cancer Normogram includes a clinical calculator to determine risk of DCIS recurrence. This calculator is based on ten clinicopathologic variables based on age, family history , surgery, radiation therapy, endocrine therapy , and pathology; however, family history was not shown to be a statistically significant predictor of recurrence [25].
Previous studies have shown epigenetic and protein changes in mammary cells obtained by random periareolar fine needle aspiration (RPFNA) from asymptomatic women at high risk for developing breast cancer even prior to the development of DCIS. A study of promoter methylation in key tumor suppressor genes that included 40 unaffected premenopausal women who underwent BRCA1/2 testing based on strong family history of breast cancer showed that women with BRCA1/2 mutations had a low frequency of CpG island promoter methylation events in key tumor suppressor genes (RARB, ESR1, INK4a/ARF, BRCA1, PRA, PRB, RASSF1A, HIN-1, and CRBP-1) whereas women without a mutation but still at high risk based on family history showed a high frequency of promoter methylation events in this same gene panel [26]. In a small pilot study of 26 similarly high-risk asymptomatic women (27 % of which had a strong family history of breast cancer), the majority of RPFNA samples with atypia (based on Masood cytology) showed high expression of key cell survival proteins when compared to non-atypical cells [27]. These studies and others shed light on the molecular underpinnings of cancer initiation and progression from premalignant to preinvasive in situ stages.
Risk Management and Therapy for DCIS in Cancer Families
The decision to undergo prophylactic mastectomy in women with a hereditary predisposition to breast cancer is fraught with emotional, social, moral, and ethical issues. The ability to give a woman a personalized breast cancer risk assessment and to better determine timing of risk-reducing surgeries is crucial. Traditional mammography has been the mainstay of screening for breast cancer in recent history; however, false-negative rates of mammography in hereditary breast cancer populations are not inconsequential. Interval cancer rates in hereditary breast cancer populations are as high as 55 % with screening mammography alone [28]. Magnetic resonance imaging (MRI) has a high sensitivity for detection of breast cancer and does not have the cumulative radiation side effect of mammography. In early studies, MRI showed a higher false-negative rate with limited sensitivity for DCIS. However, MRI imaging interpretation for DCIS has improved, and thus MRI is now recommended yearly for BRCA, TP53, and PTEN mutation carriers and for non-BRCA carriers with ≥ 20 % lifetime risk by a breast cancer risk assessment model that incorporates family history . The EVA trial compared and contrasted various breast cancer detection modalities (mammography, ultrasound , clinical breast exam, and MRI) via a prospective, multicenter observational study of 687 women at high risk for developing breast cancer over a 3-year period. Twenty-seven women were diagnosed with breast cancer during this time (including 11 cases of DCIS) with 14 of these cancers diagnosed only by MRI (52 %). Thus, cancer yield achieved by MRI alone was significantly higher with MRI’s sensitivity of 93 % and positive predictive value at 48 % compared to sensitivity of 33 % and positive predictive value of 39 % for mammography alone [28]. This study supports that MRI screening in hereditary/familial breast cancer populations can actually shift detection towards DCIS and away from invasive disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree