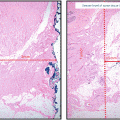
Fig. 3.1
Secretory calcifications present as large rod-like calcifications and are associated with ductal ectasia
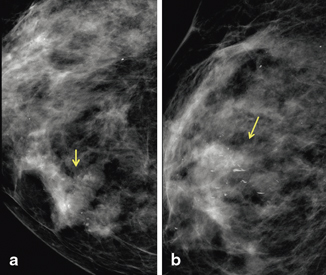
Fig. 3.2
Milk of calcium is associated with fibrocystic changes. a Cranial caudal view demonstrates amorphous-appearing calcifications. b The calcifications have a curvilinear appearance on the lateral view because they layer in the dependent portion of the microcysts
Tiny round punctate calcifications (< 0.5 mm) in a group (five or more calcifications per cubic centimeter) can be considered probably benign if no prior mammograms are available to confirm stability and if there is no know ipsilateral breast cancer. If punctate calcifications are in a linear or segmental distribution, they are suspicious and biopsy is indicated [7].
The morphology of the calcifications related to DCIS is usually fine linear or fine linear branching, fine pleomorphic, amorphous, or coarse heterogeneous. Fine linear and fine linear branching calcifications have the highest risk of malignancy with a positive predictive value (PPV) of 70 % (Fig. 3.3). The PPV of fine pleomorphic, amorphous, and coarse heterogeneous is 29, 21, and 13 %, respectively (Table 3.1) [8–12]. The fine linear and fine linear branching calcifications seen in DCIS are usually more irregular, thinner, and have a more discontinuous pattern than the benign, large, rod-like calcifications. Unlike the suspicious linear morphology, secretory calcifications may have lucent centers if the calcification is in the wall of a duct.
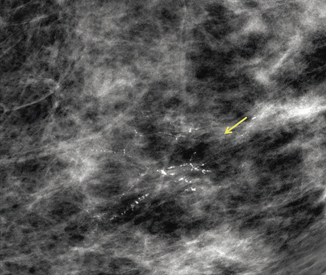
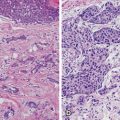
Fig. 3.3
High-nuclear-grade DCIS with comedonecrosis and microcalcifications in a 61-year-old woman presented with a palpable invasive lobular carcinoma in the upper outer quadrant. Fine linear branching calcifications in a segmental distribution are located in the lower inner quadrant, remote from the palpable finding
Table 3.1
Likelihood of malignancy as a function of BI-RADS® descriptors of calcification morphologya [8]. (2013 BI-RADS® Mammography section, Table 3.2. Reprinted with permission of the American College of Radiology (ACR). No other representation of this material is authorized without expressed, written permission from the ACR. Refer to the ACR website at www.acr.org/Quality-Safety/Resources/BIRADS for the most current and complete version of the BI-RADS® Atlas)
Morphology descriptor | Liberman et al. [9] | Berg et al. [10] | Burnside et al. [11] | Bent et al. [12] | Total |
|---|---|---|---|---|---|
Amorphous | 9/35 (26) | 30/150 (20) | 4/30 (13) | 10/51 (20) | 53/266 (21) |
Coarse heterogeneous | N/Ab | N/Sc | 1/14 (7) | 2/10 (20) | 3/24 (13) |
Fine pleomorphic | N/Ab | N/Sc | 10/34 (29) | 14/50 (28) | 24/84 (29) |
Fine linear or fine linear branching | 26/32 (81) | N/Sc | 10/19 (53) | 16/23 (70) | 52/74 (70) |
The distribution of calcifications in a segmental or linear distribution has the highest risk of malignancy, followed by grouped and regional (Table 3.2) [9, 11, 12]. Calcifications located in a line suggest malignant deposits in a duct and calcifications in a segmental distribution suggest deposits in ducts and their branches. Calcifications associated with DCIS can involve a large portion of the breast. However, diffuse, randomly distributed, punctate, and amorphous calcifications are usually benign and are usually bilateral .
Table 3.2
Likelihood of malignancy as a function of BI-RADS® descriptors of calcification distributiona [8]. (2013 BI-RADS® Mammography Section, Table 3. Reprinted with permission of the American College of Radiology (ACR). No other representation of this material is authorized without expressed, written permission from the ACR. Refer to the ACR website at www.acr.org/Quality-Safety/Resources/BIRADS for the most current and complete version of the BI-RADS® Atlas)
Distribution descriptor | Liberman et al. [9] | Burnside et al. [11] | Bent et al. [12] | Total |
|---|---|---|---|---|
Diffuse | 0/1 (0) | 0/1 (0) | 0/0 (0) | 0/2 (0) |
Regional | 6/13 (46) | 0/1 (0) | 0/9 (0) | 6/23 (26) |
Grouped | 93/254 (37) | 14/76 (18) | 19/81 (23) | 126/411 (31) |
Linear | 13/19 (68) | 8/11 (73) | 14/28 (50) | 35/58 (60) |
Segmental | 17/23 (74) | 3/8 (38) | 9/16 (56) | 29/47 (62) |
Most mammographic calcifications are benign; however, those which are malignant are usually associated with DCIS. Stomper and colleagues reviewed malignant mammographic calcifications without an associated parenchymal lesion and found pure DCIS in 65 %, DCIS with a focus of invasion in 32 %, and invasive carcinoma alone in 4 % of the cases [13].
When DCIS presents as a parenchymal lesion, with or without associated calcifications, it can have the appearance of invasive cancer. The atypical mammographic features seen in 20 % of DCIS lesions include circumscribed masses, spiculated and irregular masses, focal asymmetries, and architectural distortion. The masses may be single or multiple.
If suspicious calcifications are associated with an asymmetry or mass, there is a greater probability that the calcifications are related to invasive carcinoma or DCIS with invasive carcinoma rather than DCIS alone (Fig. 3.4). Farshid et al.’s study demonstrated that when DCIS formed parenchymal lesions without radiographically visible calcifications, it was more frequently low grade and with calcifications more frequently high grade. Periductal fibrosis and chronic inflammation resulted in the parenchymal finding. The majority of the discrete masses had a papillary component (Fig. 3.5) [4] .
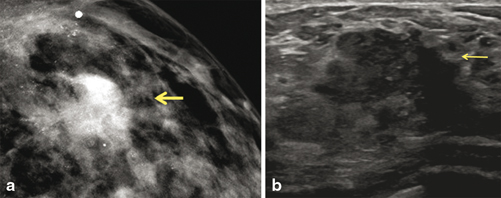
Fig. 3.4
Grade 3 invasive ductal carcinoma and high-nuclear-grade DCIS with comedonecrosis and microcalcifications, in a 39-year-old woman who presented with a self-detected breast mass. a Digital mammogram demonstrates fine pleomorphic calcifications in a regional distribution, with an associated focal asymmetry (arrow). The calcifications spanning 12 cm are located predominately in the upper outer quadrant. b Ultrasound demonstrates a 2.5-cm irregular heterogeneous mass with indistinct margins, corresponding to the palpable finding. There are echogenic foci throughout the upper outer quadrant which correspond to the calcification
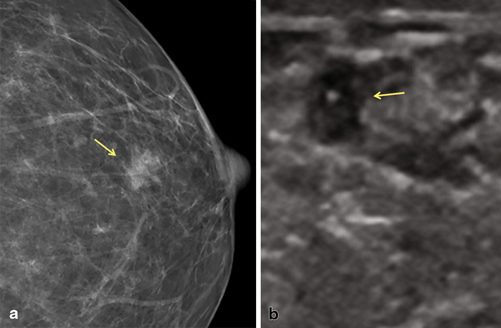
Fig. 3.5
Low-grade papillary DCIS was detected on a screening mammogram. a Digital mammogram demonstrates an irregular, indistinct isodense mass with no associated calcifications. b Focused ultrasound demonstrates an irregular, heterogeneous mass that correlates with the mammographic finding
Many studies have attempted to correlate the pathologic findings or grade with the mammographic findings of DCIS; however, a significant overlap has been found. The presence or absence of necrosis is the pathologic feature that has the most correlation with the mammographic appearance of DCIS. Calcifications associated with necrosis are more likely to be in a segmental or linear distribution than calcifications in DCIS without necrosis [14]. Barreau et al. found a relationship between the extent of the calcification seen mammographically and the grade of DCIS, with grade 3 being more extensive.
The Digital Mammographic Imaging Screening Trial (DMIST) proved digital mammography is as efficacious as film screen mammography [15]. Digital mammography has been shown to be of better and more consistent quality compared to film screen mammography, with fewer artifacts and similar dose levels [16, 17]. There has also been a higher detection rate of breast calcifications found with digital mammography compared to film screen mammography [18, 19]. With mammographic calcifications commonly being the manifestation of DCIS [20], the transition from film screen to digital mammography has demonstrated an increase in the diagnosis of DCIS [18–21]. There is criticism that the higher rates of calcifications detected can represent overdiagnosis. This criticism is based on the belief that digital screening increased the detection of DCIS that would never have presented clinically. A variety of factors including nuclear grade and the presence of comedo necrosis have been associated with the risk of DCIS progressing to invasive cancer. The higher-grade lesions have a greater risk of recurrence and tend to progress more rapidly to invasive carcinoma, although all grades of DCIS have the potential of becoming invasive (Fig. 3.6). Studies have shown that the higher DCIS detection rates are related to higher detection rates of intermediate and high-grade DCIS lesions, not low grade. In the study by Weigel and colleagues, of the 1074 screen detected cancers, 17.2 % were low grade, 37.3 % were intermediate grade, and 40.3 % were high grade [22] .
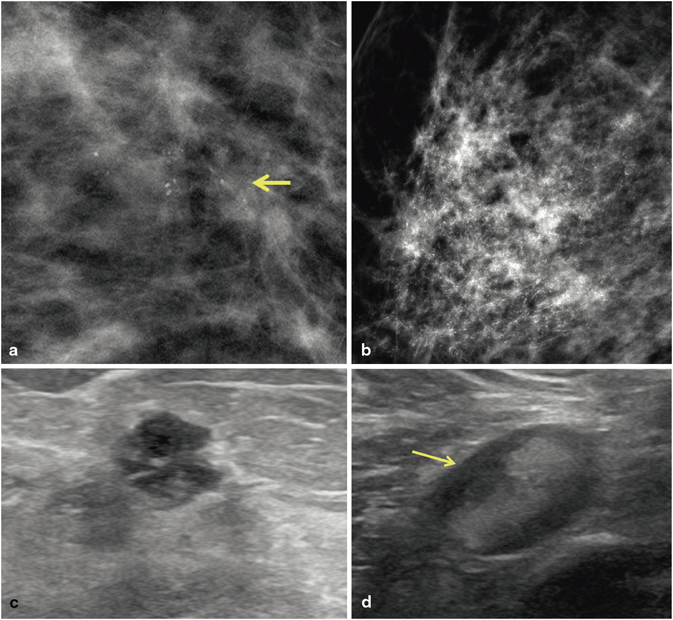
Fig. 3.6
Invasive ductal carcinoma, modified Bloom-Richardson grade 3, with high-nuclear-grade DCIS in a patient who did not follow up with the recommended biopsy. Calcifications are present in the invasive carcinoma and the DCIS. a 15 mm group of new pleomorphic calcifications were detected on screening mammogram. The patient did not come for the recommended biopsy. Fourteen months later, the patient presented with a palpable mass in the region of the previously demonstrated calcifications. b Mammogram demonstrates extensive new pleomorphic calcifications in a regional distribution spanning 8 cm. c US demonstrates a 7-mm round, hypoechoic mass with microlobulated margins. The mass contains echogenic foci consistent with calcifications. d Axillary US reveals an abnormal lymph node with compression of the fatty hilum. A US-guided fine-needle aspiration biopsy of the lymph node was positive for adenocarcinoma
All suspicious calcifications on the mammogram should be identified because the extent of the visible DCIS can affect whether breast conservation therapy (BCT) or mastectomy is recommended. It is crucial to obtain prior mammograms for comparison whenever possible, to avoid recommending removal of stable, benign calcifications. Many calcifications that would be considered suspicious if new would have a benign assessment if stability could be confirmed.
When DCIS is related to Paget’s disease, the mammogram may demonstrate subareolar or diffuse calcifications, a mass or masses, as well as skin thickening or nipple retraction. If nipple or subareolar findings are present on a mammogram, the nipple should be inspected clinically for signs of the disease. When clinical findings raise the possibility of Paget’s disease, a mammogram should be obtained to look for an underlying malignancy, although the mammogram may be normal [23] .
Digital Breast Tomosynthesis
Digital breast tomosynthesis (DBT) is a technique that uses conventional X-rays and a digital detector to obtain multiple low-dose mammographic exposures. As the X-ray tube moves in an arc over the breast, the images are obtained and then reconstructed to form thin cross-sectional images. A 1-mm slice of tissue is usually in focus with the tissue above and below the slice out of focus. This technique can eliminate the overlapping tissue that can obscure cancers and that can cause unnecessary recalls [24]. The FDA requires DBT to be combined with digital mammography (DM) for clinical use. Adding DM makes it possible to evaluate any asymmetry of the breasts and to compare prior mammograms.
Rafferty et al. showed an increase in sensitivity for invasive cancer and in situ cancers with the addition of DBT to DM; however, the increase was much smaller for in situ cancers. The majority of DCIS present as calcifications only, and no significant benefit was achieved by adding tomosynthesis for calcification-only lesions [25]. This is consistent with Skaane et al.’s study results that showed no benefit in detecting DCIS by adding DBT [26]. Spangler et al. compared detection and characterization of calcifications with DM and DBT. DM seemed to be slightly more sensitive for detecting calcifications than DBT; however, the difference was not significant. The detection of malignant and benign calcifications was slightly better with DM [27].
Studies have shown that DPT can decrease false-positive call back rates by minimizing the effect of overlapping tissues [26, 28, 29]. When two-view DBT was added to DM, a reduction in the false-positive recall rate was achieved without a negative impact on cancer recall rate [25]. The decrease in the recall rate with DBT demonstrated by Haas et al. varied by the type of lesion, and there was no change in the recall rate of calcifications and stellate lesions [30] .
The distribution of findings, especially calcifications, is also better seen on DM than DBT according to Rafferty et al. [25]. Anderson et al. reported that the distribution of calcifications was as well seen on DBT as DM; however, the morphologic details of the calcifications were not as well visualized (Fig. 3.7) [31]. Since DCIS usually presents as calcifications on mammography, visualizing the morphology and distribution of the calcifications is important for its detection.
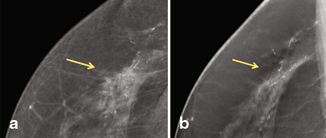
Fig. 3.7




a High–nuclear-grade DCIS with comedonecrosis and microcalcifications presented as fine pleomorphic calcifications in a segmental distribution demonstrated on digital mammogram. b The calcifications are also demonstrated on digital breast tomosynthesis; however, the morphology of the calcifications is not as well visualized
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree