RCT
RR mortality
RR node positive
HIP
0.78
0.85
Malmö
0.78
0.83
Two County
0.68
0.73
Edinburgh
0.78
0.81
Stockholm
0.90
0.82
NBSS1
0.97
1.20
NBSS2
1.02
1.09
Gothenburg
0.79
0.80
The Canadian National Breast Screening Trials, 1 and 2, were the only RCTs in which the relative risk of node-positive disease was slightly higher in the screened than in the unscreened population and the related relative risk of breast cancer mortality was close to 1 .
These early RCT trials also revealed age-specific phenomenon including a shorter sojourn time for younger women and the resultant need for more frequent screening of women in their 40s compared to women over 50. These RCT results were not without controversy, however. In 2001, in the Cochrane Collaboration review, the RCT results were each challenged as flawed, putting into question the benefit of screening mammography. Issues that were raised included the misclassification of deaths due to breast cancer or other causes and methodological flaws. In a review of the Cochrane database [11], no reduction in mortality related to mammographic screening was shown. This has subsequently been refuted by USPSTF and UK reviews confirming the significant benefit of screening mammography .
RCT Long-Term Follow-Up
The 29-year follow-up of the Swedish Two County Trial published by Tabar, 2012, was an extension of the original RCT trial. Among 133,065 women aged 40–74 years, there was a highly significant reduction in breast cancer mortality with a relative risk of 0.69 (95 % confidence interval 0.56–0.84; P < 0.0001) for women invited to screening (relative risk = 0.73 for data determined by Swedish overview committee consensus data regarding cause of death; Fig. 2.1) .
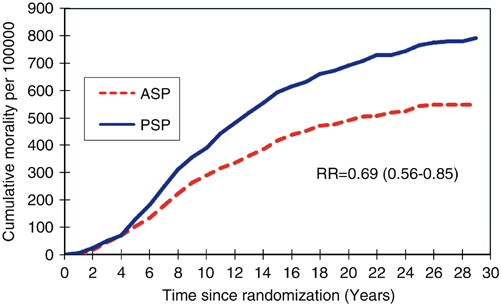
Fig. 2.1
Graph shows cumulative mortality from breast cancer according to study group as determined with local end point committee data. ASP active study population, PSP passive study population (usual care), RR relative risk. (Reprinted by permission from the Radiological Society of North America from Ref. [48])
Importantly, it demonstrated that most prevented breast cancer deaths occurred after 10 years of follow-up emphasizing that a long follow-up period is necessary to confirm a reduction in cause specific mortality. This study demonstrated that the long-term benefit of screening persisted over 29 years proving the benefit was not due to lead time bias [7]. A 25-year follow-up of the Canadian national breast screening study (CNBSS) by Miller, 2014, reported findings similar to there original study in that mammography in women aged 40–59 did not reduce mortality from breast cancer [12].
Modern Era Observational Studies
It is unlikely that studies on the scale of the early RCTs will be repeated in the current day due to the extremely large size of those trials. There have been, however, multiple observational studies evaluating women who were screened and controls, not screened with mammography [13]. Nickson et al. (2012) performed a meta-analysis of ten breast cancer observational screening studies in the modern era between the years of 1989 and 2012 in Europe and Australia. Some studies included women in their 40s. The data from the meta-analysis demonstrated odds ratios (OR) of 0.35–0.65 with a pooled OR of 0.51 for breast cancer mortality for women who underwent screening (Fig. 2.2).
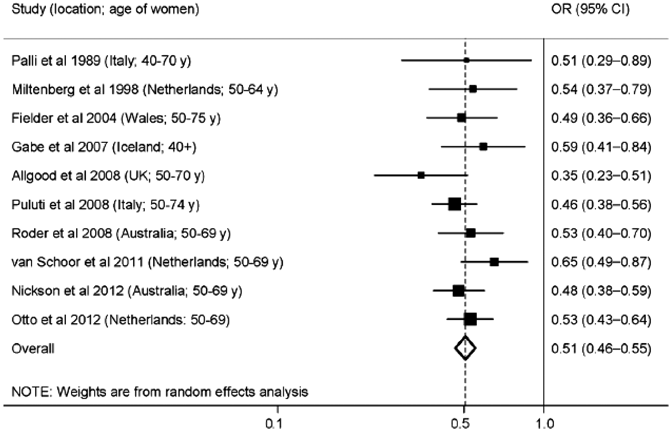
Fig. 2.2
Meta-analysis of ten case-controlled studies that have estimated the mortality benefit of screening for breast cancer. Boxes show the estimate for each study and horizontal lines show the confidence interval for each study estimate (Reprinted by permission from Ref. [13])
These results are similar to estimates of Demisse when the RCT results were adjusted for noncompliance and contamination.
A different 2012 review of published results in European, population-based, mammographic screening programs reported 38 and 48 % reductions in breast cancer deaths for women who actually were screened with mammography in incidence-based mortality studies and case-controlled studies, respectively [14]. Their interpretation of the published data was that for 1000 women screened biennially for 20 years, seven to nine women’s lives are saved. This estimates a number needed to screen (NNS) of 111–143.
Recently, two additional observational studies have demonstrated a marked decrease in breast cancer mortality with population-based screening. An Icelandic population-based service mammography screening study by Sigurdsson et al. (2013) analyzed Icelandic population-based service mammography screening of women aged 40–69, who were invited for screening at 2-year intervals. They reported a 41 % decrease in the mortality rate for all age groups in the screened group compared to the non-screened group. The screen-detected cancers were smaller, had lower tumor grade and fewer associated axillary metastases [15].
In a large multiregional case reference study in the Netherlands including 1233 cases and 2090 referent controls, Paap et al. found a 58 % reduction in breast cancer mortality in screened versus non-screened women aged 50–75 in the five regional screening organizations in the Netherlands which includes more than half of the target screening population. This result adjusted for self-selection bias of those agreeing to participate [16].
The benefit of screening mammography in decreasing late-stage disease was recently published by Foca et al. This was a temporal correlation study performed in Italy [17] evaluating the incidence of late-stage cancers (pT2–pT4) occurring in the first 8 years after a large mammographic screening program was started in 700 municipalities including 692,824 women aged 55–74 years and comparing it to the 3 years prior to initiation of screening. They demonstrated a significant decrease in the incidence of late-stage breast cancer from the third to the eighth year of screening with an incidence rate ratio of 0.71 (95 % confidence interval 0.64–0.79) at 8 years. The incidence of late-stage disease can be used as a surrogate indicator for breast cancer mortality reduction.
Helvie et al., in a deeper investigation of the effect of screening on the reduction of late-stage breast cancer, looked at the late-stage cancer incidence after adjusting for temporal incidence trends. Using estimates of temporal trends of increasing breast cancer incidence from the pre-mammography era (1977–1979) to the mammographic screening period (2007–2009) from 0.8 to 2.3 %, an average percentage change of 1.3 % correlated to a decrease in late-stage breast cancer incidence of 37 % and a reciprocal increase in early-stage disease [18].
Computer Models of the Relative Merit of Screening and Adjuvant Therapy
In 2005, the results of the National Cancer Institute (NCI) sponsored Cancer Intervention and Surveillance Modeling Network breast cancer working group study (CISNET) were published by Berry et al. “to provide estimates of the contributions of screening mammography and adjuvant treatments to the reduction in the rate of death from breast cancer among US women from 1975 to 2000” [19]. These seven computerized models created varying computer assessments using common sources of data. They reported a 28 to 65 % (median 46 %) reduction in rate of death from breast cancer attributed to mammographic screening and the remaining benefit to adjuvant chemotherapy in women aged 40 and older. The diversity of this result was felt to be related to the variable assumptions of the computer models. Prior to 1995, fewer than half of the eligible women had been screened. Even at this low screening rate, a significant mortality reduction was attributed to screening mammography.
Another analysis from the Netherlands, where mammographic screening use was 80 %, using computer simulation models evaluated the relative effects of adjuvant chemotherapy and screening mammography. They demonstrated a mortality reduction of 34 % in women aged 55–74 who were participants in the Netherlands national biannual screening program, which began in 1990. They showed that 80 % of this reduction in mortality was related to mammographic screening with approximately 20 % related to the use of adjuvant chemotherapy [20].
Controversies in Screening
There is general agreement that screening mammography saves lives with some variability between the age groups studied. Numerous US and overseas professional and public organizations have accepted and endorsed mammographic breast cancer screening above the age of 50. There are however areas of controversy in mammographic screening. The fundamental issues relate to the qualitative value comparison between lives saved or life years gained and the value of harms to women who would never develop breast cancer. Because these opinions are subjective, there will always be some differences of opinion. When economic and political constraints are added to the mix, the controversy can become intense. Typically, the controversies are mostly centered on the age to start screening and at what interval.
US Preventive Services Task Force
In 2009, USPSTF issued new guidelines for mammography screening. This organization, funded by the US government, but viewed historically as a quasi-independent, took on new heightened significance due to the impending Affordable Care Act (ACA) in 2009. Under the ACA, the USPSTF level A and B recommendations were to be funded by insurers but not level C or D. In 2009, the USPSTF recommended against routine screening for women aged 40–49 years (level C), and after public outcry added the phrasing: “The decision to start regular, biennial screening mammography before the age of 50 should be an individual one and take patient context into account including the patient’s values regarding specific benefits and harms,” but the recommendation remained a level C. The guidelines also recommended a change from annual to biennial screening mammography for women aged 50–74 years. They claimed insufficient evidence to make statements regarding screening above age 75, use of MRI, and digital mammography. A strong emphasis was placed on the harms related to screening mammography, which will be discussed in detail later. This represented an important departure from the 2002 USPSTF recommendations for screening mammography beginning at age 40 every 1–2 years. There was a rapid and passionate response to the 2009 guidelines from many branches of the medical community. The basis for the disagreement was the USPSTF’s own data showing significant benefit for screening women age 39–74 and a higher mortality reduction for annual rather than biennial screening yet the USPSTF judged the harms to outweigh the benefits. After congressional hearings, the 2009 USPSTF breast screening guidelines were rescinded from the ACA. Health and Human Services (HHS) currently uses the 2002 guidelines to comply with the ACA.
USPSTF recommendations were partially based upon an assessment of the number needed to invite (NNI) to a screening study to prevent one breast cancer death in the RCTs. A different but related metric is the NNS which is the number of women who need to actually undergo screening to prevent one breast cancer death. In the USPSTF analysis, the NNI of 1904 for women in their 40s was unacceptably high compared to women in their 50s (1339 women). Using CISNET modeling, Hedrick et al. reported that the NNS was much smaller with only 746 women aged 40–49 and overall 84 women, from aged 40–84, needing to be screened to prevent one death [21].
Cady et al. presented a surgical perspective to mammographic screening that the decrease in mortality from breast cancer and the increase of mammographic usage in the 1980s are directly related. He reports the smaller size of tumors, the decrease in the nodal metastatic involvement, and the decrease in the number of high-grade cancers with the increased usage of mammography from pre-mammography decades through 2007 are evidence of an interruption of the “progressive biological evolution of most breast cancer.” Within his analysis, states with the highest mammographic usage demonstrated a more substantial mortality decrease than states with the lowest mammographic usage. The difference has leveled out as mammographic usage has become more uniform. He reported a − 2.2 annual percentage change in age-adjusted breast cancer rates in the years 1990 through 2007, which has been projected to continue and a 30.3 % reduction in mortality from breast cancer secondary to better detection and treatment [22].
Cady addressed the USPSTF 2009 recommendations and reported a 70 % improvement in mortality reduction when screening annually in women aged 40–84 when compared to the USPSTF recommended regimen of biennial screening age 50–74. Hendrick et al. reported a 71 % improvement in mortality reduction and life years gained in a similar analysis [21].
Interestingly, a study published by Wang et al.(2014) looked at the impact of the USPSTF 2009 guidelines by analyzing utilization records from a national insurance company from 2006 through 2011 for 5.5 million women aged 40–64. They found that in the women aged 40–49, there was a brief, significant slight decrease in screening usage in the immediate 2 months following the release of the guidelines. However, there was an observed increase in screening rates for women age 40–49 and 50–64, 2 years after release of the guidelines. They acknowledged that a concurrent economic recession may have affected the early analysis; however, they looked at other screening tests in a similar time frame and did not find a drop in cervical Pap smear rates in the same 2 months, for example [23].
In a registry-based study of trends in breast cancer screening before and after the 2009 guidelines, Sprague et al. [24] looked at 150,000 women in Vermont aged 40 and over for their trends in mammography utilization. Their study demonstrated that after years of increasing screening mammographic utilization, there was a significant decline in screening with the 2009 USPSTF guidelines. The percentage of women aged 40 years and older screened in the past 1 year decreased from 45 % in 2009 to 41 % in 2011 (P < 0.01) and for those screened in the past 2 years decreased from 59.6 % in 2009 to 54.9 % in 2011 (P < 0.01). The decline was most prominent in the 40–49-year-age group. An observed decline was similarly stated in another study of the effect of the USPSTF guidelines by Sharpe et al., who demonstrated a 4.3 % drop in screening mammography utilization in the US Medicare population in the first year after the recommendations were issued. There had previously been a 1 % annual increase in mammographic utilization between 2005 and 2009. No change in mammographic utilization for women over the age of 40 after the USPSTF guidelines was reported in a different study looking at surveys of 27,829 women over the age of 40 in 2005, 2008, and 2011, before and after the guidelines were published [25].
The controversy surrounding the USPSTF guidelines is further detailed in a commentary by Martin et al. addressing the special circumstances of African-American women who are at greater risk of developing breast cancer in their 40s and at greater risk of dying from breast cancer than other women at all ages. The concern was raised that the advisement against routine screening in a woman’s 40s would be particularly burdensome to African-American women and a revision to the guidelines in this regard was proposed [26].
Mammography Screening of Women in Their 40s
Screening of women in their 40s remains controversial. The UK AGE trial was established in 1991 to specifically address the efficacy of screening mammography in women in their 40s. A total of 60,921 women were randomized with one third of the group receiving an invitation to screening and two thirds receiving usual care. After a mean follow-up period of 10.7 years, there was a 17 % reduction in breast cancer deaths for the group invited to screening. When controlled for noncompliance of those invited to screening, a 24 % reduction in mortality was observed (relative risk = 0.76, 95 % confidence interval 0.51–1.01). The concern for overdiagnosis in this younger age group was shown to be very low by Gunsoy et al. using the AGE trial data demonstrating their main analysis estimate of overdiagnosis was 0.7 % of screen-detected cancers for women in their 40s [27]. Combining the results of the entire group of RCT studies for women in their 40s demonstrated a relative risk for breast cancer mortality for women randomly assigned to mammography of 0.85 (95 % confidence interval 0.75 to 0.96), which was statistically significant, corresponding to a 15 % reduction in breast cancer mortality, favoring screening [10].
Several of the observational trials previously described included women in their 40s and within this group each demonstrated improvement in survival (Nickson, Sigurdsson, etc.). A failure analysis of the breast cancer deaths by mammographic screening history looked at 10-year cohorts of women over 40 treated at the same Harvard health care system from 1990 through 2007. For women in their 40s, the death rate from breast cancer was reported as 11.4 %. Fifty percent of breast cancer deaths occurred in women under age 50. In women between the age of 40 and 49, the study demonstrated that 70.8 % of the breast cancer deaths occurred in the 20 % of women who were unscreened [28].
2012 CISNET models for annual digital screening mammography showed an additional 1.7 lives saved and 51 life years gained per 1000 screened when the age of onset of screening was lowered from 50 to 40 [29].
A study performed by Plecha et al. in 2014 reviewed screened and non-screened women in their 40s to determine whether there was a difference with respect to the recommendations, stage at cancer diagnosis, and identification of high-risk lesions. They found that screened patients with cancer were significantly more likely to receive a diagnosis at a lower stage (stage I in 49 % of the screened group vs. 23 % of the non-screened group, P = 0.001), to have negative axillary lymph nodes (69 % in the screened group vs. 48 % in the non-screened group of DCIS and invasive cancer patients, P = 0.005) and to have smaller tumors (69 % < 2 cm in the screened group vs. 37 % in the non-screened group, P < 0.001), while non-screened patients were statistically more likely to undergo chemotherapy (44 % for the screened patients vs. 66 % for the non-screened patients, P = 0.042). Women who had high-risk lesions diagnosed in their 40s had the potential benefit of chemoprevention or screening with MRI [30].
Illness in women in their 40s has been reported to have a greater economic impact than disease in older women. For women under age 55, breast cancer resulted in the greatest productivity loss when using models relying on earnings as a measure of productivity and including costs of caregiving and household work when compared to other cancers [31].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree