Fig. 9.1
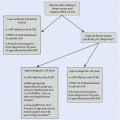
The increasing complexity of clinical decision-making in early-stage breast cancer
9.2 Multigene Assays in Invasive Breast Cancer: Prognostication
Since 2004, several MGAs have become available to clinicians treating early-stage invasive BC. This chapter discusses seven such MGAs: Oncotype DX® Breast Recurrence Score Assay (21-gene assay), MammaPrint® (70-gene panel, «Amsterdam Signature»), Prosigna® (PAM50), EndoPredict® (EP/EPclin Scores), Breast Cancer IndexSM, Insight® Dx Mammostrat® and the Breast IHC4 Assay (◘ Table 9.1).
Table 9.1
Overview of currently available multigene assays in breast cancer
Assay | Manufacturer | Tissue sample | Technological platform | Number of genes | Indication (patient/tumour characteristics)a |
|---|---|---|---|---|---|
Oncotype DX Breast Recurrence Score Assay | Genomic Health, Inc., Redwood City, CA | FFPE tissue | qRT-PCR | 21 (16 cancer-related and 5 reference genes) | HR+ HER2-negative |
MammaPrint | Agendia BV, Amsterdam, The Netherlands | Fresh frozen / FFPE tissue | Microarray | 70 | Tumour size ≤5 cm |
Prosigna (PAM50) | NanoString Technologies Inc., Seattle, WA | FFPE tissue | Hybridisation-based (for the Prosigna assay) | 58 (50 classifiers and 8 reference genes) | Postmenopausal HR+ |
EndoPredict | Sividon Diagnostics, Cologne, Germany | FFPE tissue | qRT-PCR | 11 (8 cancer-related and 3 reference genes) | HR+ HER2-negative |
Breast Cancer Index | bioTheranostics, Inc., San Diego, CA | FFPE tissue | qRT-PCR | 2 independent biomarkers: the HOXB13:IL17BR ratio and a 5-gene molecular grade index | ER+ Node-negative |
Insight Dx Mammostrat | Clarient Diagnostic Services, Inc., Aliso Viejo, CA | FFPE tissue | IHC | 5 | ER+ |
Breast IHC4 Assay | Assay is performed at local laboratories | FFPE | IHC | 4 | ER+ |
9.2.1 Oncotype DX Breast Recurrence Score Assay
The Recurrence Score assay is a quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR)-based assay performed on RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples, which was developed using three cohorts of patients with early BC and long-term follow-up [4, 5]. The assay, which is performed in a central laboratory, provides a Recurrence Score result (range, 0–100), based on expression of 21 genes (16 cancer-related, 5 reference genes), which represents a point estimate of the 10-year risk of distant recurrence [4, 5]. The Recurrence Score result is used to categorise patients into three Recurrence Score groups: low, intermediate and high (◘ Table 9.2) [4, 5].
Table 9.2
Summary of the utility of currently available multigene assays in breast cancer
Assay | Patient classification | Prognosis | Prediction of late recurrence | Prediction of adjuvant chemotherapy benefit | Prediction of neoadjuvant chemotherapy benefit |
|---|---|---|---|---|---|
Oncotype DX (Recurrence Score) | Low-risk (score <18) Intermediate-risk (score, 18–30) High-risk (score ≥31) | Yes | Yes | Yes | Yes |
MammaPrint | Low-risk High-risk | Yes | No | No | Yes |
Prosigna (PAM50)a (risk of recurrence [ROR], Prosigna score) | Node-negative: Low-risk (score, 0–40) Intermediate-risk (score, 41–60) High-risk (score, 61–100) 1–3 positive nodes: Low-risk (score, 0–40) High-risk (score, 41–100) | Yes | Yes | No | Yes |
EndoPredict (EP and EPclin scores) | EP: Low-risk (score <5) High-risk (score ≥5) EPclin: Low-risk (score <3.3) High-risk (score ≥3.3) | Yes | Yes | No | Yes |
Breast Cancer Index | Low-risk (score <5) Intermediate-risk (score, 5–<6.4) High-risk (score ≥6.4) | Yes | Yes | No | Yes |
Insight Dx Mammostrat (prognostic index) | Low-risk (score ≤0) Medium-risk (score, 0–<0.7) High-risk (score ≥0.7) | Yes | No | No | No |
Breast IHC4 Assay | Low-risk High-risk | Yes | No | No | Yes |
The assay was analytically validated [6] and then clinically validated as a prognosticator [4, 5, 7–10] according to published guidelines for biomarker validation [11, 12]. The prognostic utility of the assay in ER+ node-negative early BC patients treated with endocrine therapy alone was validated in four prospective studies, all of which used archival FFPE tissue samples: analysis of patients receiving 5 years of tamoxifen in the National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B14 study, analysis of node-negative patients in transATAC (the translational arm of the ATAC trial), the Kaiser Permanente case-control study and the Japan BC Research Group cohort study [4, 7–9]. The assay was also validated in node-positive patients (in node-positive patients in transATAC and the tamoxifen-treated arm in the Southwest Oncology Group study [SWOG] 8814) [7, 10]. Notably, the Recurrence Score result predicts late recurrence (beyond 5 years) [13], which has become clinically relevant with the recent evidence for the benefit of 10 years of tamoxifen treatment in ER+ patients [14, 15].
Several recent outcome studies with consistent results have further validated the Recurrence Score assay. The ongoing Trial Assigning Individualized Options for Treatment (TAILORx) phase III study examines the non-inferiority of endocrine treatment alone vs endocrine therapy plus chemotherapy in node-negative hormone receptor (HR) + patients with Recurrence Score results of 11–25. All patients with scores >25 received endocrine therapy plus chemotherapy, and those with scores <11 received only endocrine therapy. Only findings from the non-randomised arm with patients with results <11 have been reported thus far, and they show that these patients have excellent clinical outcomes (rate of freedom from distant recurrence at 5 years, 99.3%; overall survival at 5 years, 98.0%) [16]. The TAILORx findings are consistent with results from the endocrine therapy-only-treated patients (node-positive/high-risk node-negative) with Recurrence Score results ≤11 in the West German Study Group (WSG) Plan-B study [17]. In addition, two recently presented large cohort studies (the Clalit study and the Surveillance, Epidemiology, and End Results [SEER]-based analysis) confirm and extend the results of TAILORx and WSG Plan-B by demonstrating excellent clinical outcomes in node-negative low (<18) Recurrence Score patients [18, 19].
9.2.2 MammaPrint
MammaPrint is a 70-gene expression profile signature developed using a cohort of 78 patients <55 years with ER+, HER2-negative or HER2-positive, as well as triple-negative early BC who have had surgery, no systemic therapy, and long-term follow-up [20]. MammaPrint, which is performed by a central laboratory (in the Netherlands/USA), is microarray-based and assesses the expression of genes that regulate the cell cycle, invasion, metastasis and angiogenesis to classify patients as having either a good or poor prognosis (◘ Table 9.2) [20].
MammaPrint was first validated with fresh frozen tissue samples from the tumour bank of the Netherlands Cancer Institute [21]. Several additional validation studies (using various cohorts) followed (i.e. the TRANSBIG Consortium, which was an independent validation study, the prospective MicroarRAy PrognoSTics in Breast CancER study [RASTER] and hospital tissue banks including the Massachusetts General Hospital) [22–29]. Subsequently, the assay has also been optimised and analytically validated for FFPE tissue, and equivalence to the assay on fresh frozen tissue was demonstrated [30].
Primary analysis from the prospective MINDACT study (Microarray In Node-negative and 1–3 positive lymph node Disease may Avoid ChemoTherapy study) has recently been presented [31]. MINDACT included patients with all BC phenotypes and investigated whether patients classified as low-risk by MammaPrint can be spared chemotherapy. Only those whose MammaPrint risk assessment was discordant with their Adjuvant! Online (a tool that assesses risk based on clinical factors)-based risk assessment were randomised to receive or not receive chemotherapy. The first results from MINDACT suggest that patients with high-risk according to Adjuvant! Online and low-risk according to MammaPrint have excellent clinical outcomes without adjuvant chemotherapy [31], thus further validating (level 1A evidence) MammaPrint as a prognosticator and thus allowing women to be spared chemotherapy in 14% of the tested population.
9.2.3 Prosigna (PAM50)
The Prosigna assay, performed on RNA extracted from FFPE tissue, is based on PAM50 (a 50-gene set originally developed to classify intrinsic BC subtypes [1]) and uses a hybridisation technique to assess 58 genes (50 cancer-related, 8 reference genes) [32]. The assay can be performed locally (using the Nanostring nCounter DX Analysis System) and provides a BC intrinsic subtype based on the similarity of gene expression to prototypical expression and risk of recurrence (ROR) score (Prosigna score; range, 0–100), which is calculated based on a subset of 54 genes (46 cancer-related, 8 reference genes), a proliferation score and the tumour size. The ROR score is used to classify node-negative patients into low-, intermediate- and high-risk groups, and node-positive (1–3 positive nodes) patients into low- and high-risk groups [32] (◘ Table 9.2).
The Prosigna assay was first validated as a prognosticator using samples from ER+ endocrine therapy-treated postmenopausal patients with node-negative/node-positive early BC in the Austrian Breast and Colorectal Cancer Study Group (ABCSG)-8 trial [33]. Several additional validation studies included samples from transATAC, transATAC plus ABCSG-8, and the NCIC CTG MA2.1 trial [34–36]. The ROR score was shown to predict early (years 0–5) and late (years 5–10) recurrence in a dataset from the transATAC trial (including node-negative and node-positive patients) [37].
9.2.4 EndoPredict
EndoPredict is an 11-gene (8 cancer-related, 3 reference genes) qRT-PCR-based assay performed on RNA extracted from FFPE tissue. The assay, which can be performed by local laboratories, calculates a continuous risk score, EP (range, 0–15) [38]. When combined with nodal status and tumour size, an EP clinical score (EPclin) is calculated. The EP and EPclin scores are used to classify postmenopausal ER+ HER2-negative early BC patients who are treated with endocrine therapy into low- and high-risk groups (◘ Table 9.2) [38]. EndoPredict was validated independently using archived samples from two randomised trials (ABCSG-6 and ABCSG-8) [38]. Additional analyses on a cohort including patients from these two randomised trials who were treated with endocrine therapy for only 5 years demonstrated that EP and EPclin were predictive of both early (first 5 years) and late (years 5–10) risk of recurrence [39].
9.2.5 Breast Cancer Index
The Breast Cancer Index is a qRT-PCR-based assay performed on RNA extracted from FFPE samples. The assay is performed by a central laboratory and provides a Breast Cancer Index score, which is calculated by combining 2 signatures: a two-gene ratio, HOBXB13:IL17BR (H:I) in which the 2 genes are independent biomarkers, and a molecular grade index (MGI) that includes 5 genes related primarily to proliferation [40]. The Breast Cancer Index was developed because the MGI and the H:I ratio were shown to provide complementary prognostic information [40]. The Breast Cancer Index is a continuous score (range, 0–10) that is used to classify patients into low-, intermediate- and high-risk groups (◘ Table 9.2) [41]. The continuous score was validated in patients from the prospective Stockholm trial, an institutional cohort (University of Pittsburgh Medical Center) and in a case-control study conducted among Kaiser Permanente patients [41–43]. Analyses using patients from the Stockholm trial and the transATAC study demonstrated that the Breast Cancer Index can also predict late (years 5–10) distant recurrences in ER+ node-negative patients [44, 45].
9.2.6 Insight Dx Mammostrat
Mammostrat is an immunohistochemistry (IHC)-based assay performed on FFPE samples that measures levels of five biomarkers (SLC7A5, HTF9C, p53, NDRG1, and CEACAM5), which are independent of one another and do not directly measure proliferation or HR status. These markers were identified as the minimal panel able to predict recurrence risk in a discovery cohort and validated in node-negative ER+ patients in two initial independent cohorts [46]. The assay provides a risk score that is used to classify patients into low-, medium- or high-risk groups (◘ Table 9.2) [46]. The assay was then validated using samples from patients with ER+ endocrine therapy-treated node-negative and node-positive disease in institutional cohorts, in the NSABP B14 and B20 trials, as well as in the Tamoxifen versus Exemestane Adjuvant Multicenter (TEAM) phase III trial [47–50]. Analyses assessing the performance of the assay over time using patients from the Edinburgh Breast Conservation Series and the TEAM trial showed that the prognostic utility of Mammostrat is limited to early recurrences (first 5 years) [51].
9.2.7 Breast IHC4 Assay
The IHC4 assay evaluates levels of four biomarkers by IHC including ER, progesterone receptor (PR), Ki-67 and HER2 on FFPE samples and can be performed in local pathology laboratories. The assay provides an IHC4 score and can be combined with clinicopathological information on nodal status, tumour size, grade, age and type of endocrine treatment to provide a more powerful prognosticator (IHC4 + C) [52]. The assay was developed using patients from the transATAC study and validated as a prognosticator in a second separate cohort [52]. As in Mammostrat, an analysis assessing the performance of the assay over time (using the Edinburgh Breast Conservation Series and the TEAM trial) showed that the prognostic utility of IHC4 is only for early recurrences (first 5 years) [51]. As the assay is performed at local laboratories, known issues with precision/reproducibility of IHC testing present a challenge that has yet to be overcome, although a very recent UK study comparing four centres suggested that IHC4 + C is tolerant of variation in staining and scoring methods [53].
9.3 Prediction of Adjuvant Chemotherapy Benefit
The ability of an assay to predict adjuvant chemotherapy benefit can be determined in appropriately designed prospective trials or in archived samples from clinically relevant prospective randomised trials by using a statistical test for the interaction between chemotherapy treatment and risk group classification.
The predictive ability of Oncotype DX in ER+ early BC patients was tested using archival samples from the randomised NSABP B20 (node-negative patients) and SWOG 8814 (node-positive patients) studies [10, 54]. In both studies, high Recurrence Score patients derived a large benefit from chemotherapy (NSABP B20, 10-year distant recurrence-free [DRF] rates of 88% vs 61%; SWOG 8814, 10-year disease-free survival [DFS] rates of 55% vs 43%); low Recurrence Score patients derived minimal, if any, benefit (NSABP B20, 10-year DRF rates of 96% vs 97%; SWOG 8814, 10-year DFS rates of 64% vs 60%) [10, 54]; and the statistical test for interaction between the Recurrence Score result and chemotherapy treatment was significant. Also, in both studies, intermediate Recurrence Score patients did not derive a significant benefit from chemotherapy, although a clinically relevant effect could not be ruled out [10, 54]. The question of whether intermediate Recurrence Score patients (scores, 11–25) benefit from adjuvant chemotherapy is being addressed in the randomised arm of the ongoing TAILORx study (results are expected in 2017).
MammaPrint was evaluated in samples from a pooled study series (not a randomised trial) where tissue samples were not reanalysed [27]. The study showed that in the MammaPrint high-risk group, breast cancer-specific survival and distant disease-free survival were significantly longer for patients treated with endocrine therapy plus chemotherapy vs those treated with endocrine therapy alone, whereas for the low-risk group, this difference was not statistically significant [27]. Nonetheless, the interaction test for survival was nonsignificant [27], suggesting that the assay is not predictive. The MINDACT study (from which first results have just been presented [31]) was not specifically designed to evaluate the predictive ability of MammaPrint [55].
Mammostrat was evaluated using samples from NSABP B20; however, the interaction test was not significant [47]. The ROR was studied using samples from the prospective NCIC CTG MA2.1 trial, which randomised patients to different chemotherapy regimens including doxorubicin, cyclophosphamide and paclitaxel; dose-intense cyclophosphamide, epirubicin and fluorouracil; and dose-dense, dose-intense epirubicin, cyclophosphamide and paclitaxel (i.e. all arms involved chemotherapy treatment). The study found that the ROR was not predictive of treatment benefit; however, this study compared different chemotherapy regimens and did not have a non-chemotherapy arm [36].
In conclusion, at present, the Oncotype DX Recurrence Score assay is the only assay included as a predictive assay in international BC guidelines (◘ Table 9.3).
Table 9.3
Summary of inclusion of multigene assays in international guidelines for breast cancer treatment
St. Gallen 2015 [68] | ESMO 2015 [69] | ASCO 2016a [70] | NCCN (version 1.2016) [71] | |
|---|---|---|---|---|
Oncotype DX | Majority endorsement for prognostic (first 5 years, the panel was divided almost equally about prognostic value beyond 5 years) and predictive utility | Included as a tool that may help in treatment decisions | Panel found sufficient evidence to support clinical utility (evidence for the recommendation, high-quality; strength of the recommendation, strong) | Included as the best-validated prognostic and predictive factor |
MammaPrintb | Majority endorsement for prognostication (first 5 years only). No majority endorsement for predictive utility | Included as a tool that may help in treatment decisions | —c | Acknowledged as a clinically validated prognosticator |
Prosignab | Majority endorsement for prognostication (first 5 years and beyond). No majority endorsement for predictive utility | Included as a tool that may help in treatment decisions | Panel found sufficient evidence to support clinical utility (evidence for the recommendation, high-quality; strength of the recommendation, strong) | Acknowledged as a clinically validated prognosticator |
EndoPredict | Majority endorsement for prognostication (first 5 years, the panel was divided almost equally about prognostic value beyond 5 years). No majority endorsement for predictive utility | Included as a tool that may help in treatment decisions | Panel found sufficient evidence to support clinical utility (evidence for the recommendation, intermediate-quality; strength of the recommendation, moderate) | — |
Breast Cancer Index | Majority endorsement for prognostication (first 5 years, the panel was divided almost equally about prognostic value beyond 5 years). No majority endorsement for predictive utility | — | Panel found sufficient evidence to support clinical utility (evidence for the recommendation, intermediate-quality; strength of the recommendation, moderate) | — |
9.4 Prediction of Chemotherapy Benefit in the Neoadjuvant Setting
The ability of an assay to predict response to neoadjuvant therapy is clinically relevant, as for some patients, neoadjuvant chemotherapy may be an «overtreatment». The Recurrence Score assay was assessed in four neoadjuvant studies, in which pathological complete responses (pCR) were observed only in intermediate/high Recurrence Score patients [56–59], suggesting a role for the Recurrence Score in this setting. MammaPrint was also evaluated in four neoadjuvant studies investigating MammaPrint alone or in combination with the 80-gene BluePrint® molecular subtyping assay (Agendia), all of which demonstrated that tumours with high-risk by MammaPrint were more sensitive to neoadjuvant chemotherapy [60–63]. One study each for the Prosigna, EndoPredict, Breast Cancer Index and IHC4 assays has recently been published suggesting that these assays can also predict response to neoadjuvant chemotherapy [64–67]. No Mammostrat studies in this setting have been published to date.
9.5 Inclusion in International BC Treatment Guidelines
MGAs have been included in international BC treatment guidelines for approximately a decade. At present, Oncotype DX is included in all major international guidelines [68–71], whereas the other MGAs are included in some of them. There are also differences in the endorsement of MGAs with respect to the clinical utility of the assays (◘ Table 9.3). National guidelines also differ in their endorsement of MGAs.
9.6 Concordance of Risk Assignment by Different Tests
The increasing use of MGA in BC and the growing number of available MGAs led to an interest in comparing risk classifications on the same tumour samples using different MGAs. Several such studies have recently been published/presented, comparing Oncotype DX classification to those by MammaPrint, Prosigna and EndoPredict, as well as one study comparing three MGAs (PAM50, Oncotype DX, and IHC4) in patients from the transATAC trial (◘ Table 9.4) [34, 72–76]. All of the studies were consistent in showing differences in risk classification between the assays. Overall, Oncotype DX classified fewer patients as high-risk compared to the other evaluated assays. Also, a wide range of Recurrence Score results were observed in each of the risk classifications, as determined by the comparator MGA. Thus, these direct comparisons demonstrate that MGAs do not provide interchangeable information.
Table 9.4
Direct comparison of risk classification by different multigene assays
Study (reference) | Assays compared | Number of samples included | Main results |
|---|---|---|---|
Poulet [72] | Oncotype DX MammaPrint | 67 | In the low-risk group by MammaPrint: 66%, 31% and 3% were low-, intermediate- and high-risk by Oncotype DX, respectively In the high-risk group by MammaPrint: 45%, 50% and 5% were low-, intermediate- and high-risk by Oncotype DX, respectively |
Denduluri [73] | Oncotype DX MammaPrint | 53 | In the low-risk group by MammaPrint: 77%, 18% and 5% were low-, intermediate- and high-risk by Oncotype DX, respectively In the high-risk group by MammaPrint: 39%, 45% and 16% were low-, intermediate- and high-risk by Oncotype DX, respectively |
Alvarado [74] | Oncotype DX Prosigna | 52 | In the low-risk group by Prosigna: 79%, 18% and 4% were low-, intermediate- and high-risk by Oncotype DX, respectively In the intermediate-risk group by Prosigna : 65%, 29% and 6% were low-, intermediate- and high-risk by Oncotype DX, respectively In the high-risk group by Prosigna: 57%, 29% and 14% were low-, intermediate- and high-risk by Oncotype DX, respectively |
Varga [75] | Oncotype DX EndoPredict | 34 | In the low-risk EP score group: 82%, 18% and 0% were low-, intermediate- and high-risk by Oncotype DX, respectively In the high-risk EP score group: 26%, 35% and 39% were low-, intermediate- and high-risk by Oncotype DX, respectively In the low-risk EPclin score group: 58%, 26% and 16% were low-, intermediate- and high-risk by Oncotype DX, respectively
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|