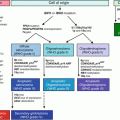
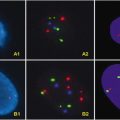
Fig. 2.1
Aspiration biopsy-reverse transcriptase-PCR analysis by Takano et al. [155] of the expression of RET, calcitonin, and CEA mRNAs. Six normal thyroid tissues samples (N), three adenomatous goiters (A), six follicular adenomas (FA), seven papillary carcinomas (P), two follicular carcinomas (FC), and 11 MTCs (M). Arrows indicate the expected positions of the PCR products
2.9 Hereditary Thyroid Cancer Syndromes
Several hereditary TC-prone syndromes have been identified, including MEN2A, MEN2B, FMTC, and hereditary PTC. Molecular diagnostic testing plays a role in the first three syndromes, where once identified in a proband, kindred analysis and subsequent early medical intervention can significantly reduce mortality and morbidity in affected individuals. Here, we will briefly discuss each syndrome.
Multiple endocrine neoplasia type 2A and 2B and familial MTC (MEN2A, MEN2B, and FMTC, respectively) syndromes are all characterized by an autosomal dominant inheritance pattern, activating RET gene mutations, and MTC. Virtually all individuals with these syndromes will develop C-cell hyperplasia followed by MTC. In fact, MTC is typically the first clinical manifestation of these syndromes, and, without medical intervention, the most common cause of death in individuals with these syndromes. Once germline RET gene mutations are identified in individuals with one of these syndromes, prophylactic thyroidectomy is recommended to prevent MTC development [159].
2.9.1 MEN2A
MEN2A or Sipple’s syndrome is an autosomal dominant disorder characterized by MTC and pheochromocytomas and hyperparathyroidism in about 50 % and 30 % of affected individuals, respectively. Other less common manifestations of MEN2A include cutaneous lichen amyloidosis and Hirschsprung’s disease [159]; 97 % of MEN2A is caused by activating missense germline RET mutations in exon 10, codons 609, 611, 618, and 620, and in exon 11, codons 630 and 634, all of which enhance receptor dimerization, with increased receptor autophosphorylation and hence increased kinase activity [139–148, 160]. Molecular diagnostic testing for MEN2A is usually directed at these specific mutations with methods described above.
2.9.2 MEN2B
MEN2B or Wermer’s syndrome is characterized by MTC, pheochromocytoma, mucosal neuromas (of the tongue, lips, conjuctivae), ganglioneuromas of the intestines, characterized by special facial appearance, and marfanoid habitus. Interestingly, the MTC of MEN2B is more aggressive than those seen in MEN2A or FMTC; 98 % of MEN2B carry RET mutations in either exon 16, codon 918, or exon 15, codon 833 [140–148, 160]. Directed testing for these mutations is the most sensitive, rapid, and accurate method for identifying individuals with MEN2B [154].
2.9.3 FMTC
MTC is the only manifestation of FMTC and typically follows a very indolent course. A kindred should have 10 or more RET mutation carriers with MTC and sufficient history to rule out pheochromocytoma or hyperparathyroidism [159]. FMTC is characterized by the same mutations as are found in MEN2A, with a few individuals having germline mutations in codons 768, 790, 791, 804, and 891 in RET exons 13, 14, and 15 [139–148, 160]. Molecular diagnostic testing is usually directed at the more frequently occurring of these mutations.
2.9.4 TC and MicroRNA Expression Profiling
Although not presently employed in TC molecular diagnostic testing, differences have been detected in the levels of specific microRNAs in PTC, ATC, MTC, oncocytic tumors, and FTC, with each histopathological TC type showing a unique microRNA expression pattern [161–164]. Additionally, the microRNA expression pattern changed with concomitant BRAF, RET/PTC, and PAX8-PPAR-gamma, but not with RAS mutations, showing possible microRNA expression pattern alterations with mutational status [164]. Although not presently employed in the molecular diagnostics of TC, different microRNA patterns are well established in TC and are likely to be incorporated into TC molecular testing in the future. Interestingly, microRNA studies have proven valuable in increasing our understanding of some thyroid lesions. For example, the hyalinizing trabecular adenoma is a distinctive thyroid neoplasm that shows some morphological features of PTC, including similar nuclear characteristics, psammoma bodies, and RET/PTC rearrangements [165–167]. Sheu et al. [168] analyzed microRNA expression and BRAF V600E mutation and RET/PTC-1 and -3 rearrangements in 18 hyalinizing trabecular adenomas. They found that microRNAs -146b, -181b, -21, -221, and -222, which are all significantly up-regulated in PTC, were suppressed in hyalinizing trabecular adenomas. Additionally, no BRAF V600E mutation and RET/PTC-1 and -3 rearrangements were detected. Based on this, the authors concluded that the hyalinizing trabecular adenoma and PTC are separate entities. The reasons for the discrepant findings of this study vs. previous studies concerning RET/PTC-1 and -3 rearrangements were not explained [167, 168].
2.10 Parathyroid Cancer: Mutations and Molecular Testing
Parathyroid cancer (PC) is a rare malignancy with an incidence of 0.4–4 % in all patients surgically treated for primary hyperparathyroidism [169, 170]. The majority of patients present with hypercalcemia, often found during a routine health screening, leading to a diagnosis of primary hyperparathyroidism. Typically, the clinical features will include high serum calcium levels (>14 mg/mL), parathyroid hormone levels at least five times above normal, a palpable lesion, bone symptoms, and operative findings suggestive of invasive growth. Histologic features of PC include broad intersecting fibrous bands within the tumor, increased mitotic index, stromal invasion, and angiolymphatic/perineural invasion. A definitive histological diagnosis of PC requires obvious tissue invasion or metastasis [169–171]. Interestingly, up to 86 % of PCs are not recognized as such intra-operatively and do not receive adequate surgical resection. In addition, in one series, half of all metastatic and recurrent PCs were initially diagnosed as benign [172, 173]. Currently there are no molecular diagnostic tests that can differentiate parathyroid adenoma (PA) from PC. Since clinically diagnosing a tumor as malignant based on metastasis is unacceptable, there is a strong need for such tests. Several areas of research appear promising.
2.10.1 Parafibromin Immunoreactivity
HRPT2 is a recently identified PC tumor suppressor found at 1q25 and has 17 exons that encode a 61 kDa nuclear protein parafibromin [174]. Germline mutations of the gene have been identified in kindreds with hyperparathyroidism-jaw tumor syndrome, which is characterized by childhood parathyroid hyperplasia, fibro-osseous jaw tumors, renal lesions, with a ~ 15 % lifetime chance of developing PC [175, 176]. Howell et al. [177] analyzed 60 parathyroid tumors , including five from individuals with hyperparathyroidism-jaw tumor syndrome, three with familial isolated hyperparathyroidism, three with MEN1, one with MEN2A, 25 sporadic PAs, 17 hyperplastic parathyroid glands, two lithium-associated tumors, and four sporadic PCs. All 17 exons and intron-exon boundaries were PCR amplified and analyzed by denaturing high-performance liquid chromotography. HRPT2 mutations were found in all four sporadic PCs, all five tumors from individuals with hyperparathyroidism-jaw tumor syndrome, and in one of the two tumors from individuals with familial isolated hyperparathyroidism. The authors hypothesized that HRPT2 mutation is an early event in PC carcinogenesis and may be a potential marker for both familial and sporadic PCs. Interestingly, parafibromin immunostaining is reduced in PCs compared to PA, with positive parafibromin nuclear staining largely excluding carcinoma and reduced staining likely indicating carcinoma or rare PAs with low staining [178, 179].
2.10.2 Cyclin D1
Cyclin D1 or PRAD1 is located at 11q13 and encodes a cyclin-dependent kinase that plays a central role in regulating and promoting G1S phase cell cycle progression [180]. Chromosomal rearrangements of the cyclin D1 regulatory region have been detected in 8 % of PAs and 20–40 % of PCS, and 31 % of hyperplastic parathyroid glands overexpress cyclin D1 [181, 182]. The overexpression appears to result from the effects of growth factor stimulation and calcium-sensing receptor activation [183].
2.10.3 Cellular Proliferation Markers
Ki-67 and PCNA immunohistochemical staining may have some value in separating PC from benign hyperplasia and adenomas, although the literature is contradictory and due to the rarity of PC most studies include relatively few cases [184, 185]. Last, the proapototic gene product FHIT appears to be preferentially mutated in PC compared to hyperplastic or adenomatous parathyroid lesions [185].
2.10.4 Chromosomal Changes
PC and PAs show gains and losses in specific chromosomal locations, often where tumor suppressor genes (at 1p, 3q, 4q, 13q, and 21q) and oncogenes are located (at 1q, 5q, 9q, 16p, 19p, and Xq). Interestingly, these areas tend to be different between PCs and PAs, likely indicating a different molecular pathogenesis of these neoplasms [186]. Presently, none of the above molecular alterations identified in PC, PA, or hyperplasia are used in molecular diagnostics, and many of these alterations appear to be quite similar between PC and PAs. Further research is required before molecular diagnostic tests for parathyroid neoplastic lesions can be implemented.
2.11 Conclusion
Over the past 20 years, different histopathologically defined thyroid malignancies have been shown to have mutation and gene expression patterns that allow them to be clearly distinguished via molecular analysis. In many cases, such analyses allow a clear diagnosis where the histology is ambiguous [1, 14]. Additionally, in many cases, as with PTC BRAF and c-MET, these mutations give useful prognostic information. Last, inhibitors for many of the constitutively active mutated kinases found in the TCs are being developed, which will eventually necessitate molecular diagnostic testing, as is currently done for KRAS mutations in colorectal carcinoma (see Chap. 5, Molecular Pathology and Diagnostics of Colorectal Cancer). Thus molecular diagnostics applied to these TCs are likely to increase. Currently, there are few molecular diagnostic procedures applied to parathyroid malignancies. However, there is a strong need for diagnostic tests that can differentiate PAs from PCs. Testing should become available as our knowledge of the molecular pathology underlying this malignancy increases.
Acknowledgement
We would like to thank Miss Jennifer Burton for her help in manuscript preparation and proofreading.
References
1.
Elsheikh TM, Asa SL, Chan JK, DeLellis RA, Heffess CS, LiVolsi VA, Wenig BM (2008) Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 130:736–744PubMed
2.
Fernandez-Ranvier GG, Khanafshar E, Jensen K, Zarnegar R, Lee J, Kebebew E, Duh QY, Clark OH (2007) Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer 110:255–264PubMed
3.
DeLellis RA (1993) Tumors of the parathyroid gland. In: Rosai J, Sobin LH (eds) Atlas of tumor pathology. 3rd series, fascicle 6. Armed Forces Institute of Pathology, Washington, DC, pp 3–63
4.
Clayman GL, Gonzalez HE, El-Naggar A, Vassilopoulou-Sellin R (2004) Parathyroid carcinoma: evaluation and interdisciplinary management. Cancer 100:900–905PubMed
5.
Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS (2009) Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 18:784–791PubMed
6.
Burgess JR, Tucker P (2006) Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid 16:47–53PubMed
7.
Colonna M, Guizard AV, Schvartz C et al (2007) A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983–2000). Eur J Cancer 43:891–900PubMed
8.
Leenhardt L, Grosclaude P, Cherie-Challine L (2004) Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 14:1056–1060PubMed
9.
Liu S, Semenciw R, Ugnat AM, Mao Y (2001) Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer 85:1335–1339PubMed
10.
Smailyte G, Miseikyte-Kaubriene E, Kurtinaitis J (2006) Increasing thyroid cancer incidence in Lithuania in 1978–2003. BMC Cancer 6:284PubMed
11.
Franceschi S, Boyle P, Maisonneuve P et al (1993) The epidemiology of thyroid carcinoma. Crit Rev Oncog 4:25–52PubMed
12.
Nagataki S, Nyström E (2002) Epidemiology and primary prevention of thyroid cancer. Thyroid 12:889–896PubMed
13.
Mizuno T, Iwamoto KS, Kyoizumi S, Nagamura H, Shinohara T, Koyama K, Seyama T, Hamatani K (2000) Preferential induction of RET/PTC1 rearrangement by X-ray irradiation. Oncogene 19:438–443PubMed
14.
Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD Jr (1995) Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 141:259–277PubMed
16.
Hancock SL, McDougall IR, Constine LS (1995) Thyroid abnormalities after therapeutic external radiation. Int J Radiat Oncol Biol Phys 31:1165–1170PubMed
17.
Mabuchi K, Soda M, Ron E, Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M, Preston DL, Thompson DE (1994) Cancer incidence in atomic bomb survivors. Part I: use of the tumor registries in Hiroshima and Nagasaki for incidence studies. Radiat Res 137:S1–S16PubMed
18.
Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M, Izumi S et al (1994) Cancer incidence in atomic bomb survivors. Part II: solid tumors, 1958-1987. Radiat Res 137:S17–S67PubMed
19.
Nikiforov Y, Gnepp DR (1994) Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphologic study of 84 cases (1991-1992) from the Republic of Belarus. Cancer 74:748–766PubMed
20.
Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, Ribeiro RC, Relling MV, Kun LE, Evans WE, Hudson MM (2003) Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med 349:640–649PubMed
21.
Hancock SL, Cox RS, McDougall IR (1991) Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med 325:599–605PubMed
22.
Knobel M, Medeiros-Neto G (2007) Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol 51:701–712PubMed
23.
Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C, Vigneri R (1992) Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med 93:363–369PubMed
24.
Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, Zhang Y, Shan Z, Teng W, Xing M (2009) Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 94:1612–1617PubMed
25.
Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, McTiernan A, Kolonel L, Yoshimoto Y, Jin F, Wingren G, Rosaria Galanti M, Hardell L, Glattre E, Lund E, Levi F, Linos D, Braga C, Franceschi S (1999) A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control 10:143–155PubMed
26.
Chen GG, Vlantis AC, Zeng Q, van Hasselt CA (2008) Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets 8:367–377PubMed
27.
Mack WJ, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, Hallquist A, Jin F, Kolonel L, La Vecchia C, Levi F, Linos A, Lund E, McTiernan A, Mabuchi K, Negri E, Wingren G, Ron E (2003) A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control 14:773–785PubMed
28.
Bosetti C, Negri E, Kolonel L, Ron E, Franceschi S, Preston-Martin S, McTiernan A, Dal Maso L, Mark SD, Mabuchi K, Land C, Jin F, Wingren G, Galanti MR, Hallquist A, Glattre E, Lund E, Levi F, Linos D, La Vecchia C (2002) A pooled analysis of case-control studies of thyroid cancer. VII. Cruciferous and other vegetables (International). Cancer Causes Control 13:765–775PubMed
29.
Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, Mack W, Mark SD, McTiernan A, Kolonel L, Mabuchi K, Jin F, Wingren G, Galanti MR, Hallquist A, Glattre E, Lund E, Linos D, Negri E (2000) A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control 11:137–144PubMed
30.
Pacini F, Elisei R, Di Coscio GC, Anelli S, Macchia E, Concetti R, Miccoli P, Arganini M, Pinchera A (1988) Thyroid carcinoma in thyrotoxic patients treated by surgery. J Endocrinol Invest 11:107–112PubMed
31.
Farbota LM, Calandra DB, Lawrence AM, Paloyan E (1985) Thyroid carcinoma in Graves’ disease. Surgery 98:1148–1153PubMed
32.
Sridama V, Hara Y, Fauchet R, DeGroot LJ (1985) Association of differentiated thyroid carcinoma with HLA-DR7. Cancer 56:1086–1088PubMed
33.
Hundahl SA, Fleming ID, Fremgen AM, Menck HR (1998) A national cancer data base report on 53 856 cases of thyroid carcinoma treated in the US. Cancer 83:2638–2648PubMed
34.
Gilliland FD, Hunt WC, Morris DM, Key CR (1997) Prognostic factors for thyroid carcinoma. A population-based study of 15 698 cases from the surveillance, epidemiology and end results (SEER) Program 1973–1991. Cancer 79:564–573PubMed
35.
Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, Ito K, Ito K, Tanaka S (1999) Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metabol 84:4043–4049
36.
Are C, Shaha AR (2006) Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors and treatment approaches. Ann Surg Oncol 13:453–464PubMed
37.
Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, Tolaney S, Holt EH, Hui P, Umbricht CB, Basaria S, Ewertz M, Tufaro AP, Califano JA, Ringel MD, Zeiger MA, Sidransky D, Ladenson PW (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373–6379PubMed
38.
Ito Y, Hirokawa M, Jikuzono T, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A (2007) Extranodal tumor extension to adjacent organs predicts a worse cause-specific survival in patients with papillary thyroid carcinoma. World J Surg 31:1194–1201PubMed
39.
Ain KB (1995) Papillary thyroid carcinoma. Etiology, assessment, and therapy. Endocrinol Metab Clin North Am 24:711–760PubMed
40.
Pelizzo MR, Merante Boschin I, Toniato A, Pagetta C, Casal Ide E, Mian C, Rubello D (2008) Diagnosis, treatment, prognostic factors and long-term outcome in papillary thyroid carcinoma. Minerva Endocrinol 33:359–379PubMed
41.
Isarangkul W (1993) Dense fibrosis. Another diagnostic criterion for papillary thyroid carcinoma. Arch Pathol Lab Med 117:645–646PubMed
42.
Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE (2006) Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30:216–222PubMed
43.
Kimura ET, Nikiforova MN, Zhu Z et al (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454–1457PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree