I
II
III
IV
Astrocytomas
Pilocytic
X
Diffuse
X
Anaplastic
X
Glioblastoma
X
Gliosarcoma
X
Oligodendroglial tumors
Oligodendroglioma
X
Anaplastic oligodendroglioma
X
Mixed tumors
Oligoastrocytoma
X
Anaplastic oligoastrocytoma
X
Ependymomas
Ependymoma
X
Anaplastic ependymoma
X
Although pathologists can classify and stratify central nervous system tumors histologically, one cannot predict the clinical outcome of a particular tumor on morphology alone. Tumors within the same histopathologic entity may behave substantially different. For example, a glioblastoma, the most common and aggressive glial tumor, can be subclassified as primary or secondary, although they are histologically indistinguishable. Primary glioblastomas arise de novo, whereas secondary arise from a pre-existing lesser grade glioma. These two subtypes should be considered as two distinct entities having unique pathogenetic pathways with different behaviors and treatment targets.
Focus has shifted to the molecular level in order to further subclassify glial tumors. Molecular alterations leading to gliogenesis can be genetic or epigenetic, resulting in altered expression of genes and their protein products. Knowledge of the molecular changes in the various types and malignancy grades of gliomas has increased dramatically over the past decade. The goal of such research is to identify biomarkers with clinical utility in establishing diagnosis, predicting prognosis, guiding treatment choices, as well as providing new cellular therapeutic targets.
1.2 Genetic Profiling
1.2.1 IDH1/IDH2
Isocitrate dehydrogenases, IDH1 and IDH2, are homologous, NADP + −dependent cytoplasmic and mitochondrial enzymes, respectively. The role of these enzymes is the conversion of isocitrate to α-ketoglutarate with the simultaneous reduction of NADP + to NADPH. IDH1 has recently been discovered to be mutated in a vast majority of astrocytic, oligodendroglial, and oligoastrocytic gliomas (WHO grades II–III), as well as in secondary glioblastomas (WHO grade IV). IDH1 mutation is very rare in primary glioblastoma and is not involved in pilocytic astrocytomas.
The most common mutation is a heterozygous point mutation with substitution of arginine by histidine at codon 132 (R132H), located in the substrate binding site. This IDH1-R132 mutation has a reported frequency of 50–93 % [2, 3]. IDH2 gene mutations affecting the amino acid R172 are much less common than the IDH1 isoform, 3–5 %, yet have been identified in a small subset of gliomas that lack the typical IDH1 mutation with a predominance in oligodendrogliomas [3]. IDH1 and IDH2 genes appear to behave dominantly and are mutually exclusive.
The carcinogenic effect caused by the mutations of IDH1 and IDH2 is not fully understood but appears to be multifactorial. The product and byproduct of the reaction, α-ketoglutarate and NADPH, both defend against cellular oxidative stress. Thus, with decreased quantities of these compounds, the cell may be more susceptible to oxidative damage. In addition to the tumorogenetic property conferred by the inability to perform the conversion, it appears that the IDH mutation confers an enzymatic gain of function. With the IDH mutations, the cancer cell has the gained ability to convert α-ketoglutarate into (R)-2-hydroxyglutarate (2HG) [4]. This reaction will not only further decrease α-ketoglutarate stores, but will also reduce NADPH to NADP+, further increasing the cell’s susceptibility to oxidative stress. The increased amount of 2HG in the brain has been associated with an increased risk of brain tumors in patients with an inborn error of 2HG metabolism [5]. Furthermore, there is an association between the IDH mutation and increased hypoxia-inducible factor-1α [6]. Hypoxia-inducible factor-1α is a transcription factor associated with carcinogenic processes, such as the upregulation of vascular endothelial growth factor, and thus promotes angiogenesis.
Mutations in IDH1 and IDH2 have also been observed in up to 22 % of acute myelogenous leukemias, which concurrently display a dramatic increase in 2HG, supporting the neoenzymatic activity [7].
IDH1 mutation has been shown to be a strong, independent prognostic biomarker not only in glioblastomas, but in diffuse gliomas of lesser grades as well [8]. There is no difference yet to be seen in terms of the point mutation, R132H versus others, regarding patient outcome [9]. While the IDH1 mutation conveys a better patient outcome, it does not predict a better response of the glioma to chemotherapy [8, 10]. In addition to its prognostic value, identification of IDH mutations could be used diagnostically. Analysis of IDH1/2 mutations could be utilized in the separation of primary and secondary glioblastomas or in the challenge of differentiating pilocytic astrocytoma from glioblastoma.
Recently, immunohistochemical staining using a specific antibody against mutant IDH1-R132H was developed, which can be applied to routine paraffin-embedded specimens. This has proved to be a tumor-specific marker differentiating reactive from neoplastic cells in grade II and III gliomas [11, 12]. In addition, this marker also identifies tumor cells in post-therapy specimens with extensive reactive gliosis. While this IHC stain is easy to use and convenient, it will neither detect the IDH mutations of other types, nor the IDH2 mutations of equal importance [11, 13]. Detection of IDH1/2 mutations can also be achieved through various polymerase chain reaction (PCR) techniques and direct sequencing.
1.2.2 MGMT Status
The MGMT (O6-methylguanine-DNA methyltransferase) gene at 10q26 encodes for a DNA repair protein. In gliomas of different grades, the MGMT gene is silenced by promoter hypermethylation, impeding transcription, and thus resulting in a decreased expression of the MGMT protein. This epigenetic modification has been associated with an increased sensitivity to alkylating chemotherapy. In alkylating therapies such as temozolamide, a methyl group is added to the O6-position of the nucleotide guanine, resulting in DNA damage and apoptosis [14]. A full-functioning MGMT would remove this methyl group; however, with reduced expression of the protein secondary to promoter hypermethylation, the cell has a decreased ability to repair alkylated DNA. Therefore, MGMT expression analysis can be used to predict which tumors may have a more favorable response to alkylating chemotherapeutic agents [15, 16]. Testing of MGMT can be applied to pediatric gliomas as well [17, 18]. MGMT promoter methylation has been found in up to 40 % of primary glioblastomas and 40–60 % of secondary glioblastomas. The aberration is also present in other diffuse gliomas, with preponderance of oligodendrogliomas at 60–93 % [19].
Although studies have shown that MGMT promoter methylation results in a significantly longer survival time for patients with glioblastoma treated with concomitant treatment of temozolomide and radiotherapy, there have been discordant reports regarding MGMT methylation as a predictor for increased survival in patients receiving radiotherapy alone [15, 20, 21]. However, in gliomas of lesser grades, there is a clear prognostic association between MGMT methylation status and sole radiotherapy [10, 22]. The underlying mechanism by which MGMT methylation would offer a favorable prognosis when not in relationship to chemotherapy is a bit more difficult to discern. As mentioned previously, gliomas often contain multiple molecular aberrancies and thus it may be the result of another molecular change, or the summation of several changes, that convey this prognostic significance to radiotherapy.
The most common method utilized to assess the MGMT promoter methylation status is a methylation-specific PCR analysis, which applies primers composed of differing quantities of CpG sites to allow differentiation between methylated and unmethylated DNA [23]. Methylation-specific pyrosequencing has also been employed with strong sensitivity [24]. Other DNA-based methods are available such as combined bisulfite restriction analysis (COBRA) and methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA). Nonmethylation-specific methods have been tested such as immunohistochemical staining, Western blotting, and biochemical enzyme assays; however, they were found to be less reliable due to staining of non-neoplastic cells, which retain MGMT expression and thus give a false result [23].
1.2.3 p53
As far as current genomic data can reveal, p53 is the most frequent genetic alteration in cancer. p53 is a tumor suppressor protein encoded by the TP53 gene located on the short arm of chromosome 17. The innate role of p53 is genomic stability achieved by cell cycle inhibition and induction of apoptosis. In activated states, p53 acts a transcription regulator leading to the upregulation of p21. The protein p21 is the stop protein responsible for binding to the cyclin-dependent kinase and inhibiting the cell from transitioning from the G1 to the S phase of cell division (see Entrez Gene). Thus, a mutated p53 will be unable to prevent cell replication resulting in uncontrolled tumor growth.
p53 is strongly associated with secondary glioblastomas and found significantly less frequently in primary glioblastomas [1].
The majority of mutations involving p53 lead to missense mutations, and there is a resultant accumulation of the protein in the nucleus of the cell. Therefore, immunohistochemical stains for p53 highlight the nuclei of tumor cells and are used as a surrogate marker for identifying cells affected by a mutation in this pathway. This methodology is more economical than PCR analysis; however, interpretation of the stain is not standardized, leading to ambiguity of interpretation [25].
The significance of the detection of p53 overexpression in gliomas is inconsistent. In terms of diagnosis, p53 would be a less favorable marker than others in distinguishing primary from secondary glioblastoma given that p53 overexpression has been reported in up to 25 % of primary glioblastomas [26]. As a prognostic marker, p53 has shown inconsistent results. While some reports indicate a shorter survival time for gliomas overexpressing p53, this finding has not been confirmed by several meta-analyses [25, 27].
1.2.4 1p/19q Deletion
The loss of chromosome arms 1p and 19q is an established genetic hallmark of oligodendroglial tumors [28]. This combined loss is detected in up to 80 % of oligodendrogliomas and up to 60 % in anaplastic oligodendrogliomas, with decreasing frequency in mixed oligoastrocytic tumors [28, 29].
The 1p/19q deletion has proven its use as a prognostic marker conveying a better response to therapy and a longer survival time [22, 29]. This co-deletion carries the same significance regardless of using chemotherapy, radiotherapy, or the combined and therefore cannot be used to guide treatment choice [22, 29]. Although there is a strong correlation between classic oligodendroglioma morphology (“fried eggs” and “chicken wire”) and the co-deletion, 1p/19q deletion cannot always be predicted by morphology alone [30].
It should be noted that deletions of the 1p arm other than the 1p/19q co-deletion have different prognostic implications. For example, a partial loss of 1p in an oligodendroglioma has been suggested to be a less favorable clinical outcome [31]. Therefore, the entire chromosomal arm should be evaluated to detect deletions of differing sizes.
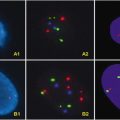
Various techniques are available to detect 1p/19q deletions; however, fluorescent in situ hybridization (FISH) is often employed due to its technical ease. FISH can be used directly on formalin-fixed paraffin-embedded tissue and does not require additional specimens from the patient. Another frequently utilized method is loss of heterozygosity (LOH), which is a PCR-based test that compares tumor DNA to the patient’s “normal” DNA, usually from peripheral blood.
1.2.5 BRAF Duplication/Fusion
BRAF, which is part of the mitogen-activated protein kinase (MAPK) pathway and located at 7q34, is aberrantly activated in a majority of pilocytic astrocytomas. BRAF is most commonly activated by duplication of the gene with subsequent fusion of the BRAF genes. BRAF aberrancies exist in 60–80 % of pilocytic astrocytomas and are rarely seen in other gliomas [32]. This discrepancy can lend utility in the differentiation of pilocytic astrocytomas and low-grade gliomas [33]. A missense mutation of BRAF, V600E, has also been identified in a minority of extracerebellar pilocytic astrocytomas; however, this mutation is more frequently seen in gangliogliomas and pleomorphic xanthoastrocytomas.
Aside from diagnostic utility, BRAF analysis may prove useful in development of therapeutic options in patients with pilocytic astrocytoma. Studies have demonstrated that silencing and inhibition of BRAF or other constituents of the MAPK pathway inhibit growth of the tumor [34].
Detection of BRAF aberrancies is often accomplished through FISH analysis with probes specific to the KIAA1549 and BRAF genes. PCR has also been utilized; however, this method may not allow for detection of variants of this fusion. Immunohistochemistry is available for detection of the BRAF V600E mutation.
1.2.6 MSH6 Mutation
Unfortunately, high-grade gliomas all too often do not respond to alkylating chemotherapeutic treatments. In these treatment-resistant gliomas, MSH6, a mismatch repair gene, has often been found to be mutated. Studies point to the alkylating chemotherapeutic agents as a cause to the somatic mutations in the MSH6 gene [35, 36]. More research into the role of MSH6 and other mismatch repair genes may be helpful in identifying therapy-resistant tumors and provide for a specific target for further treatment.
1.3 Proteomics
Genetic profiling and proteomics fall along on the same spectrum of diagnostic armory; however, they differ in detection methodologies and in clinical utility. In some cases, proteomic data will validate the established genetic alterations, while in others, proteins have been identified with little knowledge of their originating genes.
There is a wide spectrum of proteomic methodologies, ranging from a simple Western blot to 2-dimension gel electrophoresis to advanced mass spectroscopy (MS) techniques. Various MS methods have been employed, including matrix assisted laser desorption ionization—time of flight (MALDI-TOF) MS, surface enhanced laser desorption ionization—time of flight (SELDI-TOF) MS, and nanoliquid chromatography with tandem mass spectroscopy, yielding a different set of tumor proteins than provided by standard immunohistochemistry or Western blotting. The differing results may be a reflection of protein molecular weight and/or protein abundance [37, 38].
As the elucidation of genetic alterations in gliomas advances, proteomic studies will continue to reveal altered proteins. Future research endeavors into the protein-protein interactions and network-analysis may prove useful in solidifying our understanding of proteomic study results.
1.3.1 EGFR
Epidermal growth factor receptor (EGFR) gene, located on chromosome 7p12, codes for EGFR protein, a member of the transmembrane tyrosine kinase receptor family. The EGFR gene is often amplified in primary glioblastomas, up to 40 % [1, 19], resulting in over-expression of the EGFR protein. EGFR plays a role in tumorigenesis by activating MAPK and PI3K-Akt pathways, leading to cell proliferation, decreased apoptosis [39], and angiogenesis and ease of invasion [40].
Aside from overexpression of wild-type EGFR, primary glioblastomas may also harbor mutated EGFR. The most frequent rearrangement leads to a variant of the EGFR gene known as EGFRvIII (delta EGFR) [40]. This mutation is characterized by an 801 base pair (267 amino acid) in-frame deletion of exons 2–7, resulting in a truncated extracellular domain with the inability to bind a ligand. However, the receptor retains ligand-independent constitutive activity and produces tonic activation of the pathway [41]. The EGFRvIII mutation is seen in half of the glioblastoma multiforme cases exhibiting EGFR amplification, yet can also be seen as a stand-alone mutation as well [40].
The presence of EGFR amplification or EGFRvIII mutation conveys high-grade malignancy and thus may have utility as a diagnostic and prognostic tool. As such, in cases of anaplastic gliomas with equivocal histology, the detection of EGFR/EGFRvIII lends support to a higher-grade malignancy with an associated poorer patient outcome. This prognostication correlates with the finding that EGFR mutations are more frequent in primary versus secondary glioblastomas, of which primary glioblastomas are associated with later onset and aggressive clinical behavior.
Therapeutically, the EGFR pathway has provided a new target for treatment with variable success. Glioblastomas with EGFR or EGFRvIII expression have been more responsive to tyrosine kinase inhibitors most prominently when PTEN is also expressed [41].
Detection of EGFR amplification is most frequently accomplished via FISH analysis. EGFRvIII analysis is usually done via reverse transcription-PCR (RT-PCR).
1.3.2 PTEN Alterations and 10q LOH
Phosphatase and tensin homolog (PTEN), located at 10q23, is a tumor suppressor gene with an integral role in opposing the PI3K-Akt pathway. In gliomas with a mutant PTEN gene, there is an associated increase in PI3K-Akt pathway signaling, which may contribute to the tumor’s ability to invade and infiltrate. There have also been reports to support the theory that mutations in PTEN lead to activation of EGFR via a PI3K-Akt pathway demonstrating that PTEN plays a role in the angiogenesis of glioblastomas as well [42]. Mutations at the PTEN gene are found in 15–40 % of primary glioblastomas, but they are practically absent in secondary glioblastomas and other gliomas [43].
PTEN is often altered by a LOH at 10q. The LOH can occur in various sites aside from 10q23 (PTEN) such as 10q25, or 10q total loss.
PTEN mutations and 10q LOH both carry the same negative prognostication for high-grade gliomas [19]. LOH analysis or FISH can be used for evaluation.
1.3.3 Prohibitin
Prohibitin is a ubiquitous protein located at 17q21, the same location as the BRCA1 gene. Prohibitin has been found to be overexpressed in various tumor tissues, including breast, bladder, prostate, and thyroid. However, it should be noted that in gliomas there are reports of upregulation as well as downregulation of prohibitin [37, 38]. Although the exact mechanism that prohibitin may play in tumorigenesis remains unclear, given its mitochondrial location and chaperon responsibility, it is believed that the protein may have a regulatory role by modulating transcription [44]. Much more research into the mechanisms and prognostic and therapeutic values of this protein marker should be performed before detection of it is commonly employed in practice.
In summary, the knowledge gained thus far in the study of the genomics and proteomics of gliomas has allowed for both diagnostic, as well as prognostic tools when analyzing glial tissue (Tables 1.2 and 1.3). It is important to note that this is an emerging field and thus use of these evaluation methods should be used in clinical context and in reference to the rapid influx in the literature.
Table 1.2
Diagnostic molecular panels
IDH1/2 | p53 | EGFR | PTEN | |
Primary glioblastoma vs. | − | − | + | + |
Secondary glioblastoma | + | + | − | − |
IDH1/2 | BRAF | |||
Pilocytic astrocytoma vs. | − | + | ||
Higher grade astrocytoma (except 1′ glioblastoma) | + | − | ||
IDH1/2 | p53 | EGFR
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|