© Springer Science+Business Media New York 2015
Lisa A. Newman and Jessica M. Bensenhaver (eds.)Ductal Carcinoma In Situ and Microinvasive/Borderline Breast Cancer10.1007/978-1-4939-2035-8_55. Molecular Markers in DCIS
(1)
Saint Louis University School of Medicine, 3635 Vista Avenue, DT—3rd Floor, 63110 St. Louis, MO, USA
(2)
Department of Surgery, Saint Louis University School of Medicine, 3635 Vista Avenue, DT—3rd Floor, 63110 St. Louis, MO, USA
Keywords
DCISMolecular subtypesEstrogen receptorHer2/neu p53p16Ki-67Biological markersIntroduction
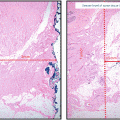
Ductal carcinoma in situ (DCIS) includes a heterogeneous group of noninvasive breast malignancies with a variable potential for evolution. It is characterized by a proliferation of malignant epithelial cells that are confined to the duct without invasion through the basement membrane into the surrounding stroma [1]. When it was initially recognized as a distinct clinical entity during the first half of the twentieth century, DCIS accounted for only 1–2 % of newly diagnosed breast cancers. Since it generally presented as a large palpable mass, mastectomy was accepted as the standard of care and was routinely curative [2]. Due to improvements in both quality and consistency of mammographic screening, the incidence of DCIS has continued to increase and it now accounts for approximately 20 % of all breast cancer diagnoses [3]. DCIS is also able to be detected at an earlier stage with overall smaller tumor burden. These advances have led to the widespread use of breast conservation therapy for women with DCIS who are appropriate candidates. Early studies conducted following the institution of breast-conserving surgery for DCIS showed a local recurrence rate of approximately 25 % in women who did not undergo adjuvant radiation therapy with half of these recurrences presenting as invasive disease [4–8]. Although the use of adjuvant radiation and endocrine therapy have substantially decreased the local recurrence rate in these women, our understanding of the pathophysiology of DCIS and the factors involved in its progression to invasive disease is still unclear. Developing a mechanism that can better classify DCIS subtypes that are more likely to progress to invasive disease would greatly enhance individualization of management strategies by assisting in both reducing overtreatment of less concerning lesions and identifying those patients who should be treated more aggressively. The study of the biologic features and molecular markers of DCIS that could offer accurate prognostic information and ultimately provide treatment guidelines is of paramount importance.
Natural History
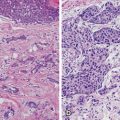
The natural history of breast cancer involves progression of normal breast tissue to invasive carcinoma due to accumulation of somatic mutations involving key growth, differentiation, and cell communication pathways. The initiation of breast cancer is due to transforming events in a single cell with point mutations, copy number alterations, epigenetic modifications, and general genome instability being frequently associated with disease progression [9, 10]. DCIS is thought to be the immediate precursor of invasive disease based on molecular and pathological studies [11, 12]. Information on the specific functional events that drive progression of DCIS to invasive carcinoma is limited. Numerical grading systems have been developed to reflect tumor differentiation and growth by assigning points for specific cellular features of the tumor. Although no accepted standard method of grading is available for DCIS, there are three overall grades used that describe the tumor cells according to the degree to which they resemble normal breast cells. The grades commonly used are well differentiated (grade 1), moderately differentiated (grade 2), and poorly differentiated (grade 3) DCIS.
Progressive growth of DCIS results from alterations in normal growth-regulating mechanisms [13]. A molecular biological marker , or biomarker , is an objective measurement of a normal biological response. Biomarkers most commonly associated with breast cancer include the hormones estrogen and progesterone and their respective receptors, the oncogene erbB2 (HER2/neu), the tumor suppressor proteins p16 and p53, and the tumor proliferation marker Ki-67. Although numerous histopathological characteristics have been identified as predictors of recurrence of DCIS, including lesion size, nuclear grade, architectural pattern, margin status, and presence of comedonecrosis, finding molecular markers to further target the malignancy in question and guide risk stratification at the time of diagnosis is paramount [14]. Understanding these biomarkers and their predictive value for both treatment response and potential disease recurrence could help individualize treatment plans to optimize patient outcomes.
Estrogen and Progesterone
Estrogen and progesterone are steroid hormones that stimulate the development and maintenance of female breast tissue. Estrogen exposure has been established as a risk factor for future breast cancer development. It has been hypothesized that estrogen acts as a stimulatory hormone, thereby increasing the frequency of mitotic activity within the breast with malignant phenotypes developing due to errors in cell division [15]. The estrogen and progesterone receptors (ER and PR) are commonly present on breast tumors with approximately 70 % of all DCIS being ER positive [16]. ER positivity is more often seen in well-differentiated and moderately differentiated DCIS, whereas it is reported to be present in only 25 % of poorly differentiated lesions [17–19].
Expression of ER, and to a less common extent PR, is routinely assessed on pathological specimens of DCIS in order to predict clinical response to hormonal therapy, such as selective estrogen receptor modulators (SERMs). SERMs, namely Tamoxifen and Raloxifene, are competitive inhibitors of estrogen binding to the estrogen receptor . The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 study evaluated the use of Tamoxifen compared to placebo in 1804 women with DCIS undergoing breast-conserving surgery followed by radiation therapy. After 5 years of treatment, women in the Tamoxifen arm had fewer breast cancer events (8.2 vs. 13.4 %, p = 0.0009) than did those on placebo [19, 20]. In a Cochrane review from 2012 including both NSABP B-24 and the UK/ANZ Trial 2011, Staley et al. showed that Tamoxifen after breast-conserving surgery for DCIS reduced recurrence of both ipsilateral DCIS (hazard ratio {HR} = 0.75) and contralateral DCIS (relative risk {RR} = 0.50) as well as ipsilateral invasive disease (HR = 0.79) and contralateral invasive disease (RR = 0.57). Despite this reduction in recurrence seen with Tamoxifen use, this review did not detect a difference in all-cause mortality (RR = 1.11) [21].
In order to investigate whether or not ER status plays a role in breast cancer recurrence, Kerlikowske et al. evaluated biomarker expression and risk of local recurrence among 329 patients who underwent lumpectomy alone for DCIS between 1983 and 1994. With a median follow-up time of 8 years, their univariate analysis suggested that ER-negative status was associated with DCIS recurrence (RR = 1.7) [22]. In a study from Vienna published in 2004, Roka et al. analyzed 132 patients who were treated by breast conservation between 1978 and 2001. Rates of in-breast tumor recurrence were significantly higher in ER-negative disease compared to ER-positive disease (12.2 vs. 3.7 %, p < 0.04) [23]. Newer studies will need to be performed in our current era of more homogeneous treatment strategies and pathological evaluation of DCIS to further elucidate the prognostic value of ER status.
Her2/neu Gene (erbB2 Oncogene)
Her2/neu amplification status is routinely assessed in invasive breast cancer as a predictor of responsiveness to both standard chemotherapy and targeted monoclonal antibody therapy. In addition to the steroid hormone receptors, the Her2/neu gene is one of the most thoroughly investigated biomarkers in the study of DCIS. It is a member of the epidermal growth factor receptor (EGFR) family and Her2/neu has the strongest tyrosine kinase activity of all EGFRs. Activation of Her2/neu leads to an increased rate of cell survival, cell proliferation, and tumorigenesis [24].
Her2 overexpression has been shown to be associated with increased cell motility and has been localized to portions of the cell membrane actively engaged in cell migration [25]. It is thought that this increase in cell motility could increase the extent of DCIS within the breast. DePotter et al. demonstrated a statistically significant relationship between Her2 overexpression and extent of DCIS. This group showed that 67 % of Her2-negative DCIS spanned less than 1 cm, whereas only 17 % of Her2-positive DCIS were this small [25]. Her2/neu overexpression has also been consistently associated with higher grade DCIS [26] and is amplified more frequently in DCIS than in invasive disease [27–29]. Wei et al. reported a 64.3 % rate of Her2 overexpression in pure DCIS, compared to only 42.5 % in invasive disease [29]. This emphasizes the importance of determining the prognostic capabilities of Her2 status in women with DCIS.
Multiple studies have investigated the local recurrence rates in Her2-positive DCIS. Holmes et al. analyzed 141 patients who underwent lumpectomy alone for DCIS between 1983 and 2002. With a median follow-up of 10 years, 60 recurrences were noted (42.6 %). Through multivariate analysis, Her2 positivity was the only pathological characteristic that was statistically significantly associated with disease recurrence (p = 0.28) [30]. Kerlikowske et al. also showed on univariate analysis that Her2 positivity was associated with DCIS ipsilateral recurrence (HR = 2.0) [22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree