Chapter 39 Microsporidiosis
Microsporidia is a nontaxonomic designation used to refer to a group of obligate, intracellular parasites belonging to the phylum Microsporidia.1,2 The Microsporidia are ubiquitous organisms that are emerging pathogens in humans. They are most likely zoonotic or waterborne infections (or both).3 In the immunosuppressed host (e.g., those treated with immunosuppressive drugs or infected with the human immunodeficiency virus (HIV), particularly at advanced stages of these diseases) microsporidia can produce a wide range of clinical diseases. While the most common manifestation is gastrointestinal tract infection; encephalitis, ocular infection, sinusitis, myositis, and disseminated infection are also described. These organisms have also been reported in immunocompetent individuals.
In 1857 Nosema bombycis, a parasite of silkworms, was the first organism identified as belonging to the order Microsporidia,1,4 and in 1959 Microsporidia were identified as etiologic in human infection.5 The Microsporidia are important agricultural parasites in insects, fish, laboratory rodents, rabbits, fur-bearing animals, and primates.1,4,6 They have been described in dogs and birds kept as household pets.6,7 In their hosts most infect the digestive tract, although reproductive, respiratory, muscle, excretory, and nervous system infections have been documented.4,6,8,9
The phylum Microspora contains more than 1100 species distributed into at least 150 genera, of which the following have been demonstrated to cause human disease (Table 39-1)4,6,8,9: Nosema (N. corneum renamed Vittaforma corneae10 and N. algerae renamed Bracheola algerae)11 Pleistophora, Encephalitozoon, Enterocytozoon,12 Septata13 (reclassified as Encephalitozoon),14 Trachipleistophora15,16 and Brachiola11 (recently reclassified as Anncaliia).17 In addition, the genus Microsporidium has been used to designate Microsporidia of uncertain taxonomic status.6 Vittaforma corneae,18 Encephalitozoon cuniculi,4 Encephalitozoon hellem,19 Trachipleistophora hominis,15 and Encephalitozoon intestinalis20,21 have been cultivated in tissue culture systems in vitro. Enterocytozoon bieneusi has not been cultivated continuously in vitro, although limited in vitro cultivation has been reported.22 Adenovirus can mimic the cytopathologic effect of Microsporidia.23 N. salmonis (Nucleospora), an organism related to Ent. bieneusi, can be grown in vitro.24,25 Experimental infection of simian immunodeficiency virus (SIV)-infected rhesus monkeys with Ent. bieneusi from human tissue has been demonstrated.26
Table 39-1 Microsporidia Identified as Pathogenic to Humans
| Identified in Patients with AIDS | |
| Identified in Other Patients | |
Enc. hellem has been associated with superficial keratoconjunctivitis, sinusitis, respiratory disease, prostatic abscesses, and disseminated infection.4,6,8,9 Enc. cuniculi has been associated with hepatitis, encephalitis, and disseminated disease.27–30 Encephalitozoon (Septata) intestinalis is associated with diarrhea, disseminated infection, and superficial keratoconjunctivitis.6,13,31 Nosema, Vittaforma, and Microsporidium have been associated with stromal keratitis associated with trauma in immunocompetent hosts.9,18 Pleistophora, Brachiola, and Trachipleistophora have been associated with myositis.6,11,15,32–34 Trachipleistophora has been associated with encephalitis and disseminated disease.15,16,35 Ent. bieneusi, originally described in humans,12 is associated with malabsorption, diarrhea, and cholangitis.6,36
GENERAL CHARACTERISTICS
The Microsporidia are true eukaryotes, containing a nucleus with a nuclear envelope, an intracytoplasmic membrane system, chromosome separation on mitotic spindles,37 and a Golgi apparatus.38 Microsporidia lack mitochondria and centrioles and have prokaryote-size ribosomes39,40 lacking a 5.8S ribosome subunit but having sequences homologous to the 5.8S region in the 23S subunit.41 The small subunit rRNA of several Microsporidia have been sequenced and found to be significantly shorter than both eukaryotic and prokaryotic small subunit rRNA.40,42 These rRNA genes are in a subtelomeric location on each chromosome of Enc. cuniculi43,44 and lack the paromomycin binding site.45 Sequence data of rRNA from the Microsporidia have been used to develop diagnostic polymerase chain reaction (PCR) primers and in the study of phylogenetic relations (reviewed by Weiss and Vossbrinck42 and Weiss46). The karyotype of several members of the phylum Microspora has been determined by pulsed field electrophoresis. The genome size of the Microsporidia varies from 2.3 to 19.5 Mb.6 The genomic size of the Encephalitizoonidae is less than 3.0 Mb, making them among the smallest eukaryotic nuclear genomes so far identified.43,47,48
Microsporidia are currently classified on the basis of ultrastructural features, including the size and morphology of the spores, number of coils of the polar tube, developmental life cycle, and the host–parasite relationship.1,49,50 Molecular analysis of rRNA genes has begun to alter this classification system.42,51 Antigenic differences between Microsporidia demonstrable by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis52,53 have been used as adjunctive evidence when determining phylogenetic relationships among the Microsporidia infecting humans.14,53 Molecular phylogenetic data indicates that the Microsporidia are related to fungi and are not “primitive eukaryotes”.54–58 Keeling has suggested that the Microsporidia are a sister group to the Zygomycota.59
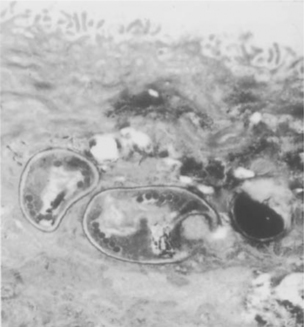
Microsporidia form characteristic unicellular spores (Fig. 39-1) that are environmentally resistant. Enc. cuniculi spores remain viable for 6 days when in water and 4 weeks when dry at 22°C.60 Nosema bombycis spores may remain viable for 10 years in distilled water. Spores may be killed by exposure for 30 min to 70% ethanol, 1% formaldehyde, or 2% Lysol or by autoclaving at 120°C for 10 min.60 Whereas Microsporidia spores can be as large as 12 mm, the Microsporidia infecting humans have spores that range from 1 to 4 mm. The spore coat consists of an electron-dense, proteinaceous exospore, an electron-lucent endospore composed of chitin and protein, and an inner membrane or plasmal-emma.6,61 A defining characteristic of all Microsporidia is an extrusion apparatus that consists of a polar tube attached to the inside of the anterior end of the spore by an anchoring disc; depending on the species, it forms four to ∼30 coils around the sporoplasm in the spore. During germination the polar tube rapidly everts, forming a hollow tube that brings the sporoplasm into intimate contact with the host cell. The polar tube provides a bridge to deliver the sporoplasm to the host cell. The mechanism by which the polar tube interacts with the host cell membrane is not known, but it may require the participation of host cell proteins such as actin.62 It is possible that the sporoplasm interacts with the host cell membrane as it emerges from the polar tube. If a spore is phagocytosed by a host cell, germination occurs and the polar tube can pierce the phagocytic vacuole, delivering sporoplasm into the host cell cytoplasm. The overall process of germination and formation of the polar tube inoculates the sporoplasm directly into a host cell, functioning essentially like a hypodermic needle.63–65
Conditions that promote germination vary widely among species, presumably reflecting the organism’s adaptation to its host and external environment.6,66,67 Conditions that promote spore discharge include pH shifts,68 dehydration followed by rehydration,69 various cations70 and anions,71 mucin or polyanions,72 hydrogen peroxide,67,73 ultraviolet radiation, and the calcium ionophore A 23187.74 Inhibitors of spore discharge include magnesium chloride, ammonium chloride, low salt concentrations, sodium fluoride, ultraviolet light, temperatures higher than 40°C, calcium channel antagonists, calmodulin inhibitors,73 cytochalasin D, demeclocyline, and itraconozole.73 Regardless of the stimuli required for activation, all Microsporidia appear to exhibit the same response to the stimuli (i.e., increased intrasporal osmotic pressure). This results in an influx of water into the spore accompanied by swelling of the polaroplasts and posterior vacuole prior to spore discharge. In Brachiola (Nosema) algerae, it has been proposed that activation brings trehalose in contact with the enzyme trehalase, causing increased osmotic pressure.66,75–78 The polar tube then discharges from the anterior pole of the spore in an explosive reaction, occurring in less than 2 s. It has been suggested that the hollow tube is formed by a process of eversion, similar to everting the finger of a glove.63
EPIDEMIOLOGY
Although initially regarded as rare, Microsporidia are now believed to be common enteric pathogens that are self-limited or asymptomatic in normal hosts.79–81 Reported prevalence rates among those with acquired immunodeficiency syndrome (AIDS) have varied between 2% and 70% depending on the symptoms of the population studied and the diagnostic technique employed.6,8,9 Co-infection with different Microsporidia or other enteric pathogens can occur. Asymptomatic carriage can occur in immunocompromised patients. Enc. cuniculi isolates from various animal species have been identified and separated based on the number of tetranucleotide repeats (59GTTT39) in the intergenic spacer region of their rRNA genes.82 Differences have also been found in the intergenic spacer region of rRNA genes of Ent. bieneusi.83–85 Such differences have proven useful for epidemiologic identification of the environmental source of Microsporidia isolated from humans.
Serosurveys in humans have demonstrated a high prevalence of antibodies to Enc. cuniculi and Enc. hellem, suggesting that asymptomatic infection may be common.86,87 Serologic cross-reactivity among Microsporidia has been demonstrated by immunofluorescence88 and Western blotting.89 Singh et al90 found positive titers in six of 69 healthy adults in England, 38 of 89 Nigerians with tuberculosis, 13 of 70 Malaysians with filariasis, and 33 of 92 Ghanians with malaria. In another study 14 of 115 travelers returning from the tropics and none among 48 nontravelers were seropositive.91 In a study of HIV-positive male subjects, 10 of 30 were seropositive and all had traveled to the tropics.92 Antibodies to Enc. intestinalis were found among 5% of pregnant French women and 8% of Dutch blood donors87 and antibodies to Enc. hellem among 5.3% of HIV-positive Czech patients.93 These data suggest that Microsporidia are common in humans and, similar to other gastrointestinal pathogens, are associated with travel or residence in the tropics or developing nations.
It is likely that microsporidiosis is a zoonotic infectious disease.3 Encephalitozoon is a widely distributed parasite of mammals and birds, and the onset of microsporidiosis has been associated with exposure to livestock, fowl, and pets.81 Encephalitozoon has been described in lovebirds and budgerigars (parakeets),7 and one case of Enc. hellem has been reported in a patient who had two pet lovebirds.94 Up to 30% of dogs in animal shelters may excrete microsporidia in their stools, and Enc. cuniculi was etiologic in the death of Maltese puppies in a recent outbreak.81,95 Encephalitozoon was found in the stools of many animals in an epidemiologic survey in Mexico. Ent. bieneusi has been reported in pigs,96 dogs, birds,97 and SIV-infected rhesus monkeys.98 Nosema, Brachiola and Vittaforma infections are believed to be due to traumatic inoculation of environmental spores of insect pathogens into human tissue.34
It has been possible to transmit Encephalitozoon via rectal infection in rabbits, suggesting that sexual transmission may also occur.99 Enc. hellem has been demonstrated in respiratory tract mucosa as well as the prostate and urogenital tract of patients, raising the possibility of respiratory and sexual transmission in humans. Although congenital transmission of Enc. cuniculi has been demonstrated in rabbits, mice, dogs, horses, foxes, and squirrel monkeys, no such congenital transmission has been demonstrated in humans.100,101 It is possible that many of the microsporidia are also water-borne pathogens. Enterocytozoonidae such as Nucleospora (previously Enterocytozoon) salmonis102 have been found in fish,103 and many of the human pathogenic Microsporidia have been detected in surface water supplies.104–108
MICROSPORIDIAN INFECTION IN HIV-UNINFECTED PATIENTS
Levaditi et al. suggested in 1923 that microsporidians could be associated with human disease.109 This was proven in 1973 when a 4-month-old athymic male infant died with severe diarrhea and malabsorption; at autopsy microsporidia (N. connori) were discovered in the lungs, stomach, small and large bowel, kidneys, adrenal glands, myocardium, liver, and diaphragm.110 Disease due to Encephalitozoon was suggested by positive immunoglobulin G (IgG) and IgM indirect immunofluorescence assays (using Enc. cuniculi) in a 3-year-old boy with seizures and hepatomegaly.86,91 Encephalitozoon sp was also reported in a 9-year-old Japanese boy with headache, vomiting, spastic convulsions, and recurrent fever.5 Encephalitozoon sp have been found in the stools of patients in surveys for the etiology of diarrhea in less developed countries.111 Ent. bieneusi has been identified as a cause of self-limited diarrhea in immunocompetent hosts.79,112–116 It is seen in 1–10% of children in developing countries presenting with diarrheal syndromes,117–121 and in patients undergoing renal, liver or bone marrow transplantation.122–127 A Pleistophora sp was identified in 1985 in skeletal muscle of an HIV-negative patient with myositis and has also been described in the skeletal muscle of HIV-positive patients.8,32,33,128 Brachiola algerae infection of the skin with dissemination has been seen in a patient with leukemia129 and in a woman with rheumatoid arthritis on antibody to TNF-α.34,130
Two cases of corneal microsporidiosis due to Microsporidium africanus in Botswana and Microsporidium ceylonesis in Sri Lanka were described in 1973 and 1981, respectively.131,132 Additional cases of microsporidian keratitis have been identified in immunocompetent hosts.6 One of these organisms was classified as N. occularum,132,133 and the other, which was successfully propagated in vitro, was named N. corneum134 (now V. cornea).10 Among immunologically normal patients with corneal infections, one patient required enucleation,131 one underwent unsuccessful penetrating keratoplasty,135 one was successfully treated with a corneal transplant,132 and the last was maintained on a variety of topical agents without effect until keratoplasty.134
MICROSPORIDIAN INFECTION IN AIDS PATIENTS
Microsporidia were recognized as opportunistic pathogens causing diarrhea and wasting in AIDS patients in 1985.12 Since then, although most reported cases have still involved diarrhea, the spectrum of diseases caused by these organisms has expanded to include keratoconjunctivitis, disseminated disease, hepatitis, myositis, sinusitis, kidney and urogenital infection, ascites, cholangitis, and asymptomatic carriage.6,8,81
In patients with HIV infection evaluated for diarrhea the prevalence of microsporidiosis has ranged from 7% to 50%.136–143 Weber et al examined 1271 stool specimens from 845 HIV-infected patients in Switzerland and found Microsporidia in eight of 88 patients with chronic diarrhea, three of 57 patients with self-limited diarrhea, and none among 700 asymptomatic patients.8 Rabeneck et al in a prospective study, found microsporidia in 29% of patients seen at an HIV primary care clinic, but there was no association with the presence of diarrhea (18/55 with diarrhea and 13/51 without diarrhea had microsporidia on duodenal biopsy).144,145 Patients with microsporidiosis in this study had a mean CD4+ T-lymphocyte count of 113 cells/mm3. It is thus likely, as is true for other gastrointestinal protozoa, that asymptomatic carrier states exist. During a 15-month follow-up of this study, diarrhea developed in two of the 13 asymptomatic patients and continued in the 18 symptomatic patients.146 Kotler and Orenstein, in a study of patients presenting to a gastroenterology clinic, found that 39% of patients with HIV and diarrhea (55/141) had microsporidiosis and that the presence of microsporidia was associated with wasting, a mean CD4+ T-lymphocyte count of 28 cells/mm3, and abnormal D-xylose tests.147 In contrast, only 2.6% of HIV patients without diarrhea (1/38) had microsporidiosis, and this patient subsequently developed diarrhea on follow-up. Coyle et al.148 using the polymerase chain reaction (PCR) employing primers to rRNA gene of Ent. bieneusi, found that 37% (25/68) of HIV patients with diarrhea and 2.3% (1/43) of HIV patients without diarrhea had microsporidiosis. The one asymptomatic patient had an abnormal D-xylose test. Based on these studies microsporidia appear to demonstrate strength of association, coherence, and reproducibility with respect to being etiologic for a diarrheal syndrome. Further evidence of the association of microsporidia with diarrhea is provided by the utility of albendazole in the treatment of microsporidian infection. Therapy with albendazole results in cure of diarrhea associated with the elimination of Enc. intestinalis from the stools of infected patients149; treatment with fumagillin has a similar effect in patients with Ent. bieneusi infection.150,151
ENTEROCYTOZOON sp
The major syndrome associated with microsporidiosis is diarrhea and wasting. This is usually due to Ent. bieneusi (more than 90% of cases in the United States) and occasionally Enc. intestinalis (in Europe this organism may be a more frequent cause of diarrhea).21 Ent. bieneusi occurs most commonly when CD4+ T-lymphocyte counts are less than 50 cells/mm3; it presents with chronic nonbloody diarrhea, anorexia, weight loss, and bloating without associated fever. Although originally thought to invade only enterocytes, it has been demonstrated that Ent. bieneusi can also invade cholangioepithelium.152 When present in the cholangioepithelium this organism has been associated with sclerosing cholangitis, AIDS cholangiopathy, and cholecystitis. Interestingly, an Ent. bieneusi-like organism has been identified in SIV-infected rhesus monkeys as the etiologic agent of cholangitis and hepatitis.98,153 Systemic dissemination does not appear to occur with Ent. bieneusi. One case report described this organism in nasal mucosa154 and four other reports have described pulmonary involvement due to Ent. bieneusi,155 however, such cases may represent contamination due to spores from gastrointestinal secretions. Reports indicate that Ent. bieneusi may cause self-limited infections in immunocompetent patients.3,79 Other intestinal pathogens may occur simultaneously or sequentially with this or any other microsporidian.
This parasite has a unique intracellular developmental life cycle.156 A characteristic feature of this organism is the presence of electron-lucent inclusions demonstrating a lamellar structure. These inclusions are closely associated with the nuclear envelope or endoplasmic reticulum (or both). The earliest intraepithelial stages of the parasite are rounded proliferative cells limited by a typical unit membrane in direct contact with the host cell cytoplasm. In these cells nuclear division is not immediately followed by cytokinesis, thus resulting in the production of multinucleate proliferative plasmodia. After the production of multiple nuclei the parasites form electron-dense disk-like structures that cluster in stacks of three to six, eventually forming the coiled portion of the polar tube. When these multinucleated sporagonial plasmodia divide by invagination of the plasmalemma, multiple spores are formed. In mature spores the polar tubule has five to seven coils that appear in two rows when seen in cross sections by transmission electron microscopy.
ENCEPHALITOZOON sp
Encephalitozoon sp are widely distributed in many animals.81 Three members of the family Encephalitozoonidae have been associated with disease in humans: Enc. cuniculi, Enc. hellem, and Enc. intestinalis (previously known as Septata intestinalis). It appears that these microsporidia have the capacity to disseminate widely in their hosts, and involvement of most organs by these organisms has now been documented.6,8,29,157
Enc. intestinalis has been associated with diarrheal disease,13 cholangitis,158 keratoconjunctivitis, osteomyelitis of the mandible,159 and disseminated infection149,160 in AIDS patients. The ability of this parasite to disseminate correlates with its ability to grow in many cell types both in vivo and in vitro. Elimination of this parasite from patients with diarrhea treated with albendazole correlates with the resolution of symptoms.149,161 The parasite develops in the cytoplasm of intestinal enterocytes, macrophages, fibroblasts, and endothelial cells of the lamina propria and in epithelial cells of the kidney and gallbladder.13 Sporogony is tetrasporous, and tubular appendages originate from the sporont surface and terminate in an enlarged bulb-like structure. Unlike other Encephalitizoonidae, Enc. intestinalis-infected cells have a unique parasite-secreted fibrillar network surrounding the developing organisms, so the parasitophorous vacuole appears septate. Mature spores in cross section have a single row of four to seven coils of polar tubules.
Enc. cuniculi has been associated with hepatitis,162 peritonitis,163 hepatic failure,28 disseminated disease with fever,27,28,157 renal insufficiency, and intractable cough.164 These infections have been reported to respond to albendazole.27,157,164 Granulomatous encephalitis due to Enc. cuniculi was first described in rabbits in 1922, and cases of encephalitis and seizures due to Enc. cuniculi have been reported in AIDS patients.29 Enc. cuniculi was identified in cerebrospinal fluid, sputum, urine, and stool specimens from a 29-year-old man with a CD4+ T-lymphocyte count of 0 cells/mm3, and he had enhancing lesions demonstrable by both computed tomography (CT) and magnetic resonance imaging (MRI) of the brain that diminished when albendazole was administered.29
Enc. hellem has been reported to cause disseminated disease associated with renal failure, nephritis, pneumonia, bronchitis, and keratoconjunctivitis.165–168 This organism is also recognized to cause infection of the nasal epithelium and is an important etiologic agent in cases of sinusitis in patients with HIV infection. Among the ocular infections due to Encephalitozoonidae reported in the literature, most have been attributed to Enc. hellem, including three cases originally classified as due to Enc. cuniculi.9,169 The remaining cases have been due to Encephalitozoon sp or Enc. intestinalis.170 Ocular microsporidian infection in HIV-1-infected patients has been restricted to the superficial epithelium of the cornea and conjunctiva (i.e., superficial keratoconjunctivitis) and has rarely progressed to corneal ulceration. Similar microsporidian infections are reported in contact lens wearers.171 Patients present with bilateral punctate epithelial keratopathy and conjunctival inflammation resulting in redness, foreign body sensation, photophobia, and changes in visual acuity. Slit-lamp examination usually demonstrates punctate epithelial opacities, granular epithelial cells with irregular fluorescein uptake, conjunctival injection, superficial corneal infiltrates, and a noninflamed anterior chamber.
Enc. hellem has been cultured in vitro from the urine of patients with disseminated microsporidiosis172 and those with keratoconjunctivitis.53,173 As this organism has been found in the lung, it has been suggested that respiratory spread of this microsporidian may occur.167 Due to the presence of this organism in the urogenital tract including the prostate, and the ability to transmit this organism by rectal inoculation in rabbits, it is possible that Enc. hellem may be acquired as a sexually transmitted disease.174 Enc. hellem and Enc. cuniculi have similar developmental life cycles.37 The genus is characterized by the presence of a phagosome-like parasitophorous vacuole. Nuclei of all stages are unpaired. Meronts divide repeatedly by binary fission. Sporonts divide into two sporoblasts that mature into spores. No tubular appendages or fibrillar networks are produced, as is seen in Enc. intestinalis.In cross section the mature spore has five to seven coils in single rows (Fig. 39-2).
OTHER MICROSPORIDIA
Trachipleistophora hominis is a pansporoblastic microsporidian that has been described in several patients with disseminated disease in the setting of AIDS.15 The organism has been cultivated in vitro. Trachipleistophora anthropophthera infection has presented as encephalitis, myositis, and keratoconjunctivitis.16,35,175 Several of these patients responded clinically to albendazole. Brachiola vesicularum caused myositis in an HIV-1-infected patient and responded to a regimen of albendazole and itraconozole.11 Cases of myositis due to Pleistophora sp and Brachiola algerae have also been reported in AIDS patients.32–34176
DIAGNOSIS
Diagnostic tests for patients with suspected microsporidiosis are described in Table 39-2. In general, a urine examination should be done whenever microsporidiosis is considered, as microsporidia associated with disseminated disease such as Encephalitozoon and Trachipleistophora have invariably been present in such specimens when examined. As gastrointestinal disease with Ent. bieneusi is not associated with disseminated disease, this organism is not seen in the urine. For gastrointestinal disease, examination of three stools with chromotrope and chemofluorescent stains is often sufficient for diagnosis. If the stool examination is negative and the diarrhea is chronic (more than 2 months duration), endoscopy should be performed.
Table 39-2 Diagnosis of Microsporidiosis
| Test | Utility |
|---|---|
| Urine examination | This is often positive in cases of microsporidiosis due to microsporidia other than Enterocytozoon and should be done in all suspected microsporidia cases. |
| Stool examination | This is useful for gastrointestinal presentations. At least three stools should be examined. The combination of chromotrope and chemofluorescent stains provides the highest sensitivity and specificity. |
| Endoscopy | This should be considered for all patients with chronic diarrhea of more than 2 months’ duration and negative stool and urine examinations. In this group endoscopy has yielded a diagnosis of microsporidia presence in up to 30% of patients. Tissue should be examined by a microsporidial stain. Touch preparations are useful for rapid diagnosis (within 24 h). If microsporidia are demonstrated to invade the lamina propria, the urine examination should be repeated, as Encephalitozoon are the most likely etiologic agent. In this setting albendazole has high efficacy. |
| PCR | This test is available as a research technique. Species identification can be performed on stool, urine, and tissue samples. |
| Electron microscopy | This identifies the species of microsporidia involved and is crucial for identification of new species. It is essential for the characterization of microsporidia in unusual or new locations. |
| Conjunctival scrapings | This has a high diagnostic yield in microsporidian keratoconjunctivitis. Urine examination should also be performed in suspected cases to screen for disseminated microsporidiosis. |
| Nasal scrapings | This can be useful for the diagnosis of microsporidian sinusitis. As most of the microsporidia associated with sinusitis are present in the kidneys, urine examination should be routine for suspected sinusitis cases. If these tests are negative, biopsy of nasal mucosa may be useful for diagnosis. |
| Serology | This is not useful for diagnosis but may be useful for epidemiologic surveys. |
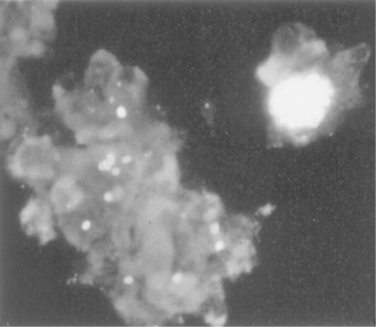
Effective morphologic demonstration of microsporidia by light microscopy can be accomplished by staining methods that produce differential contrast between the spores of the microsporidia and the cells and debris in clinical samples (e.g., stool) in which they are found. In addition, given the small size of the spores (1–5 mm) adequate magnification (i.e., 1000X) is required for visualization. Chromotrope 2R,177 calcofluor white (fluorescent brightener 28),178 and Uvitex 2B179 have been reported to be useful as selective stains for microsporidia in stool specimens and other body fluids (Fig. 39-3). The chromotrope 2R-based method of Weber et al177 is similar to a standard trichrome stain, but the chromotrope 2R concentration is 10-fold higher and the staining time is longer. Further modifications of this method by Ryan et al.180 using aniline blue in place of fast green, and Kokoskin et al.181 using an elevated temperature, are preferred by some laboratories. Using this stain, spores appear as 1–3 mm ovoid light pink structures with a belt-like stripe girding them diagonally and equatorially against a green or blue background. Spores can also be visualized by ultraviolet (UV) microscopy using chemofluorescent optical brightening agents such as Calcofluor white M2R (fluorescent brightener 28, Fungi-Fluor) and Uvitex 2B (Fungiqual A; Dieter Reinehr and Manfred Rembold, Spezialchemikalien fur die Medizinische Diagnostik, Kandern, Germany), which stain chitin in the spore wall (endospore layer).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree