Metastatic Prostate Cancer
INCIDENCE
Prostate cancer is the most commonly diagnosed cancer in men and a leading cause of cancer death. In the United States, there were approximately 238,590 new prostate cancer cases and 29,790 deaths in 2013 (1). Incidence and mortality rates for prostate cancer are highly variable worldwide.
Prostate cancer incidence in the United States increased steadily throughout the second half of the 20th century. This increase appears related to the increase in life expectancy and associated rise in number of older men at risk for prostate cancer. Other factors including widespread use of prostate-specific antigen (PSA) screening also appear to have contributed to the increase in annual prostate cancer incidence. PSA screening identified a large number of prevalent cases of asymptomatic prostate cancer. The annual incidence of prostate cancer peaked at approximately 350,000 cases in 1993. After declining in the late 1990s, the annual incidence of prostate cancer has slowly increased. In contrast to the striking variations in annual rates of prostate cancer diagnosis, prostate cancer mortality rates have declined since 1990.
SPECTRUM OF ADVANCED DISEASE
Prostate cancer preferentially spreads to regional lymph nodes and bone. Clinically significant metastases to liver, lung, or other visceral organs are less common. Bone scans using technetium-99m methylene diphosphonate 99mTc MDP) are routinely used to assess skeletal involvement. Computed tomography scans or magnetic resonance imaging scans are used to assess regional lymph nodes.
The spectrum of advanced disease has markedly changed in recent decades. Prostate cancer screening with serum PSA has been accompanied by a dramatic stage migration, and now less than 10% of men in the United States have radiographic evidence of metastases at initial diagnosis. Additionally, PSA testing is routinely used for surveillance after surgery or radiation therapy for early stage disease. As a result, advanced prostate cancer now includes men with rising serum PSA levels as the only indication of disease progression after prior treatment for early stage disease.
ANDROGEN DEPRIVATION THERAPY
 HYPOTHALAMIC-PITUITARY-GONADAL AXIS
HYPOTHALAMIC-PITUITARY-GONADAL AXIS
Prostate cancers ubiquitously express the androgen receptor and require androgens for growth and survival. Testosterone synthesized by the Leydig cells of the testis is the primary source of androgens in men. Leydig cell synthesis of testosterone is regulated by luteinizing hormone (LH) of pituitary origin. The release of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. Levels of circulating testosterone are maintained within normal limits by negative feedback at the level of the hypothalamus and pituitary.
Approximately 98% of plasma testosterone is present in a biologically inactive protein bound form. Free testosterone enters cells by diffusion across the cell membrane. In some tissues, including the brain, pituitary, and kidney, unmodified testosterone is bound by the androgen receptor. In other tissues, including the prostate, seminal vesicles, epididymis, adrenals, liver, and skin, testosterone is efficiently converted to dihydrotestosterone (DHT) by membrane-bound 5α-reductase type II. DHT binds the androgen receptor with approximately threefold greater affinity than testosterone. In adipose tissue, testosterone is converted to estradiol by cytochrome p450-dependent aromatization.
Under the influence of pituitary adrenocorticotropin (ACTH), the adrenal glands produce androstenedione, dehydroepiandosterone (DHEA), and dehydroepiandosterone sulfate (DHEA-S). These compounds, collectively known as the adrenal androgens, have relatively weak androgenic activity. Androstenedione can be converted to testosterone in peripheral tissues and in the prostate. In normal men, the adrenal cortex is a minor source of androgen production. Residual adrenal androgens, however, may be important in promoting disease progression in men with advanced prostate cancer following medical or surgical castration.
 GnRH AGONISTS AND ANTAGONISTS
GnRH AGONISTS AND ANTAGONISTS
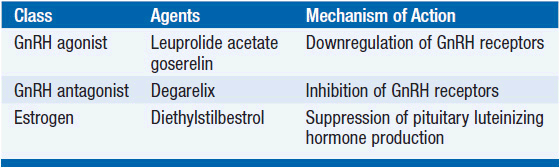
The mainstay of treatment for metastatic prostate cancer is androgen deprivation therapy (Table 41-1). Permanent androgen deprivation can be accomplished by bilateral orchiectomies. Reversible methods of androgen deprivation therapy include diethylstilboestrol (DES), GnRH agonists, and GnRH antagonists. DES suppresses pituitary LH production resulting in castrate testosterone levels. Administration of GnRH agonists causes an initial stimulation of pituitary LH production and rise in serum testosterone levels. Chronic administration of GnRH agonists causes downregulation of pituitary GnRH receptors and prompt suppression of testicular androgen production. GnRH antagonists directly inhibit pituitary GnRH receptors, producing castrate testosterone levels without an initial rise.
The various forms of androgen deprivation therapy have similar efficacy. A meta-analysis of 10 randomized controlled trials, for example, concluded class Agents Mechanism of Action GnRH agonist Leuprolide acetate Downregulation of GnRH receptors goserelin GnRH antagonist Degarelix Inhibition of GnRH receptors Estrogen Diethylstilbestrol Suppression of pituitary luteinizing hormone production that bilateral orchiectomies and GnRH agonists produce equivalent progression-free and overall survival (2). GnRH agonists have become the most prevalent form of androgen deprivation therapy because they are convenient, are potentially reversible, and lack the psychological implications of bilateral orchiectomies. DES is not routinely used because it causes increased risk of thromboembolic events.
Androgen deprivation therapy achieves prompt marked decline in PSA in nearly all cases and symptomatic improvement in most men with disease-related symptoms. For men with bone metastases, the median time to disease progression is 12–18 months and median survival is 24–30 months. In men without radiographic evidence of metastases, response duration and survival are substantially longer. The median survival for men with receiving adjuvant therapy for regional lymph node metastases, for example, is greater than 10 years.
For men without clinical or radiographic evidence of metastases, the optimal timing of androgen deprivation therapy is controversial (3). Three randomized controlled trials have compared immediate versus delayed androgen deprivation therapy for men with locally advanced disease or regional lymph node metastases. Early primary androgen deprivation therapy improved survival for men with locally advanced nonmetastatic prostate cancer. Adjuvant androgen deprivation therapy improves survival for men with locally advanced prostate cancer treated with radiation therapy and men with lymph node-positive prostate cancer treated with radical prostatectomy and pelvic lymphadenectomy. The effects of early androgen deprivation therapy on clinical outcomes for men with “PSA-only” disease are unknown.
Most adverse effects of androgen deprivation therapy are due to severe gonadal steroid deficiency. These adverse effects include loss of lidido, hot flashes, fatigue, and osteoporosis. Androgen deprivation therapy also increases fat mass and decreases insulin sensitivity. Consistent with these adverse effects, androgen deprivation therapy is associated with greater risk for diabetes. Some but not all studies have linked androgen deprivation therapy to greater risk for myocardial infarction.
 COMBINED ANDROGEN BLOCKADE
COMBINED ANDROGEN BLOCKADE
Nonsteroidal antiandrogens including bicalutamide, flutamide, and nilutamide competitively inhibit the binding of testosterone and DHT to the androgen receptor. These nonsteroidal antiandrogens bind to the androgen receptor with <2% of the affinity of DHT. Monotherapy with nonsteroidal antiandrogens is inferior to castration for metastatic prostate cancer, likely due to their relatively low binding affinity.
The combination of androgen deprivation therapy with an antiandrogen, termed combined androgen blockade, has the potential advantage of inhibiting testicular androgen production and blocking the action of residual adrenal androgens. Three meta-analyses concluded that combined androgen blockade is associated with a small survival benefit compared to androgen deprivation therapy alone (4), although combined androgen blockade is also associated with greater side effects than androgen deprivation alone.
MANAGEMENT OF CASTRATION-RESISTANT DISEASE
Most men with metastatic prostate cancer experience disease progression within a few years of initiating androgen deprivation therapy—a disease state termed castration-resistant prostate cancer. Rising serum levels of PSA are usually the first indication of disease progression.
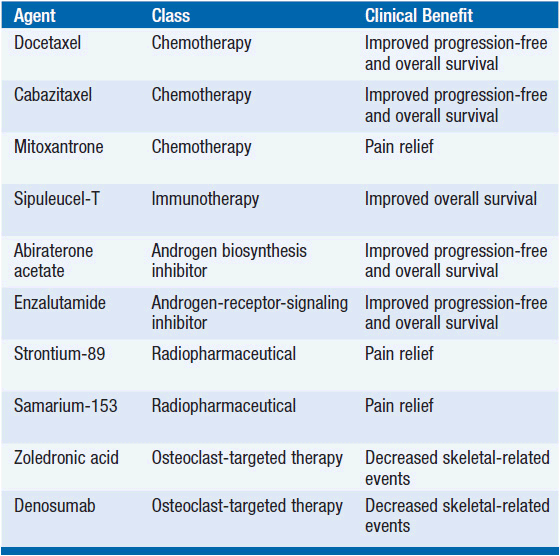
The landscape of clinical management for castration-resistant prostate cancer has changed dramatically in recent years. Currently, there are five therapies (docetaxel, cabazitaxel, sipuleucel-T, abiraterone acetate, and enzalutamide) approved by the United States Food and Drug Administration for metastatic castration-resistant based on improved overall survival. A randomized controlled trial of radium-223 reported survival benefit in men with metastatic castration-resistant disease. Additional therapies are approved based on prevention or relief of symptoms (Table 41-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree