Metastatic Breast Cancer | 6 |
Katherine H. R. Tkaczuk, Paula Rosenblatt, Angela DeRidder, Syed S. Mahmood, Reshma L. Mahtani, Geetha Pukazhendhi, Susan B. Kesmodel, Jason Molitoris, and Randi Cohen
Despite an increase in detection of early-stage breast cancer over the past 30 years, approximately 5% to 10% of patients diagnosed with breast cancer present with de novo metastatic disease; in addition, up to 30% of lymph node (LN)-negative and 70% of LN-positive patients will eventually develop metastases (1,2). The primary goals of systemic treatment for MBC are palliation of symptoms, maintenance and improvement in quality of life, and prolongation of survival. These goals must be balanced against toxicities associated with treatment.
The 5-year survival of MBC has increased from 5% to 10% in 1990 to 26% presently based on Surveillance, Epidemiology, and End Results (SEER) data (3,4). In a series from MD Anderson, the median overall survival (OS) for patients with de novo stage IV and relapsed disease was 39.2 and 27.2 months, respectively (P < .0001) (5). Improved survival among patients with recurrent or newly diagnosed metastatic disease has been attributed to more aggressive management and the availability of effective therapeutics (6,7).
This chapter reviews the initial assessment and management of MBC. We review hormonal therapy (HT), combination hormonal and targeted therapy, single agent chemotherapy, and combination chemotherapy. Finally, while systemic therapy is the mainstay of treatment for MBC, there are certain situations in which radiation and surgery may play prominently in management for palliation of symptoms, maintenance and improvement in quality of life, and prolongation of survival. These situations are discussed at the end of the chapter.
INITIAL ASSESSMENT
At the diagnosis of metastatic disease, initial assessment should include:
• Tissue diagnosis of invasive breast cancer with markers (estrogen receptor [ER], progesterone receptor [PR], and HER2) is mandatory before systemic therapy is considered. We prefer to rebiopsy metastatic sites before final recommendations are made as a change in receptor status can alter treatment decisions in 20% of cases (8).
• History and physical exam. MBC patients who are being considered for systemic chemotherapy should be carefully evaluated in terms of clinical symptoms, physical exam (PE), and social support. The PE should include assessment of vital signs, performance status (PS), and comprehensive clinical exam. These assessments should continue with each prechemotherapy exam. Low PS is a predictor of poor survival, increased toxicity, and decreased chemotherapy response (9).
• Laboratories. Routine prechemotherapy labs should include a complete blood count (CBC) and comprehensive metabolic panel (CMP). Tumor markers (CA 15-3, 27.29) and circulating tumor cells (CTCs) can be considered but should never be used as a sole assessment of response to therapy. CTCs have prognostic significance (10).
• Imaging. We recommend baseline staging within 4 weeks of initiation of new systemic therapy and we do not routinely stage the central nervous system (CNS) unless there are specific symptoms to suggest CNS involvement. We recommend to stage with contrast-enhanced CTs of chest/abdomen/pelvis + bone scan or fludeoxyglucose (FDG) PET with diagnostic CTs initially.
• Genomic tumor tissue testing. Genomic tumor tissue testing looks to identify what may account for the differences in response to treatment and to guide use of therapies that target the tumor’s specific genes, proteins, or the tissue environment that contributes to growth and survival. At present, treatments using these approaches are still considered investigational and are being examined in multiple ongoing clinical trials (NCI-MATCH/EAY131 Trial, NCT02465060). We do not routinely send genomic analysis of tumor for newly diagnosed metastatic patients.
Choice of Therapy
The choice of treatment for MBC is influenced by several factors including hormone receptors, estrogen-ER, progesterone-PR (ER/PR) and HER2-neu status, presence of symptoms from disease, presence of visceral crisis, PS, prior receipt of adjuvant treatments, number of prior treatments for metastatic disease and presence of residual side effects of therapy, response to prior treatments, duration of response, time to treatment failure/progression, and comorbidities (11). As the goal of treatment for MBC is quality of life, if a patient is eligible for HT, this is chosen as first-line treatment. Earlier studies have shown response to HT to be a predictive factor for rate and duration of response to chemotherapy (12). Patients with symptomatic visceral disease (liver, lymphangitic lung, bone marrow) involvement should be considered for upfront chemotherapy regardless of ER/PR status, as chemotherapy is more likely to offer rapid relief of symptoms. Single agent and combination therapies are discussed in the following.
Follow-Up
Close follow-up is important in all patients with MBC. Patients receiving hormonal/endocrine therapy (HT) will be seen monthly and then visits spaced depending on the pace of their disease. Our approach is to see our MBC patients on chemotherapy approximately every 2 to 4 weeks depending on the treatment and schedule. We do not see our weekly chemotherapy patients every week, unless toxicities are reported, and nursing visits are done in between MD visits. We feel that this schedule of follow-up allows adequate clinical assessments and assessment of toxicity to therapy. A thorough history with assessment for changes in PS, weight loss, fatigue, gastrointestinal symptoms, neuropathy, depression, and distress should be performed at each visit. A complete PE with specific documentation of abnormalities should be completed and labs should be monitored for cytopenias, kidney function, and liver function.
Assessment of Response to Therapy
• Imaging should be completed every two to four cycles of chemotherapy in the absence of clinical symptoms and signs of progression. We adhere to the RECIST 1.1 criteria for assessment of response and changing therapies (13). We do not recommend routine imaging with PET/CT scans. While we often obtain a baseline PET/CT to determine the location of metastatic lesions, we typically restage and follow disease status with contrast-enhanced CTs and bone scans.
• Tumor markers (CA15-3 or CA 27.29) are optional in follow-up of patients with MBC. We do not recommend any changes to systemic therapy solely based on rising or declining levels of these tumor markers. If tumor markers were not initially elevated, we do not continue to check routinely.
• CTC enumeration is not routine in our assessment of response to therapy. We do utilize CTCs for the purpose of assessment of tissue markers (ER, HER2), in particular when tumor tissue may not be available, not accessible for biopsy, or in cases of bone only disease when there is a concern about reliability of the immunohistochemical (IHC) stains for ER or HER2 after bone decalcification.
DURATION OF THERAPY
The optimal duration of systemic chemotherapy is unknown and must be individualized. Benefit of therapy must be balanced against the toxicities and effects on quality of life. Patients with ER/PR+ disease can be switched to maintenance HT, while patients with HER2+ disease can continue with anti-HER2 therapies +/− HTs without chemotherapy after best response is achieved with chemotherapy. Patients with triple-negative breast cancer may come off palliative chemotherapy and take a “chemotherapy holiday” at the time best response is achieved and monitored closely for increased burden of disease and new symptoms. Clinical trials should be considered and discussed with patients early in the treatment of MBC.
HORMONE THERAPY FOR METASTATIC BREAST CANCER
Indications for Endocrine (Hormonal) Therapy in MBC
Initial assessment of the patient with ER/PR+ MBC is crucial in determining the optimal treatment strategy. The landscape of hormonal treatment is vast and includes ovarian suppression, selective estrogen receptor modulators (SERMs), selective estrogen receptor degrader (SERD), aromatase inhibitors (AI), mammalian target of rapamycin (mTOR) inhibitors, and cyclin-dependent kinase (CDK) 4/6 inhibitors. Choosing the optimum strategy takes into account menopausal status, location and extent of disease, and history of prior adjuvant HT. In the latter case, the duration of disease-free or treatment-free interval is relevant and may influence the choice of the next hormonal agents or transition to chemotherapy, as possible resistance mechanisms or primary or secondary hormone insensitivity need to be considered if the patient is not responding to HT.
The former approach that all visceral disease in MBC requires upfront chemotherapy treatment is no longer supported for clinical practice unless the disease burden is large, causing symptoms, rapidly progressing, or there is evidence of end-organ dysfunction (visceral crisis due to extensive involvement of the liver, lymphangitic lung involvement, or bone marrow involvement with cytopenias) where immediate response is required.
A high-quality systematic review in 2003 assessing randomized clinical trials compared frontline HT to chemotherapy and showed no difference in OS (hazard ratio [HR] 0.94; P = .5), albeit a statistical significance for overall response rate (ORR) (relative risk 1.25; P = .04) did favor chemotherapy, while treatment-related toxicities were notably increased in the chemotherapy arms (14). Furthermore, there is no benefit from combining HT with chemotherapy in ER/PR+ HER2-negative MBC (15,16).
ER or PR Positivity and Response to Therapy
ER and/or PR prognostic and predictive values have been demonstrated in the metastatic setting. The presence and degree of tissue ER/PR expression strongly predicts response to hormonal treatments, with responses seen in approximately 60% of patients with both ER+ and PR+ tumors, versus 30% in patients with either ER+ or PR+ status alone, versus fewer than 10% of women with receptor negative (ER−/PR−) disease (17,18). Patients with both ER+ and PR+ MBC have been shown to have a more favorable prognosis with longer OS than their counterpart single ER or PR+ tumors. This was assessed in an analysis of three phase III trials of AIs according to ER and PR status, showing that although there were no differences in clinical benefit, the median OS of women with ER+/PR+ tumors was significantly longer than those with single ER or PR+ tumors (800 vs. 600 days, P = .01) (19). Additionally in women with ER+ tumors, the median OS of those with tumors that were also PR+ was significantly longer than those that were PR− (800 vs. 625 days, P = .02) (19).
DURATION OF HORMONAL THERAPY
Tumor response assessments while on HT should be similar to other therapies. However, ER/PR+ disease is more often associated with bone only disease, which is not considered evaluable/non-measurable by RECIST 1.1 criteria (13). HT should be continued until there is clear evidence of disease progression or new lesions. Some patients with ER/PR+ MBC may experience prolonged periods of disease stability (years) while HT is continued and quality of life is preserved. Our approach is to proceed with first disease assessment/restaging no sooner than 3 to 4 months into HT, especially if the patient has bone only metastasis. During this period of time we follow patients clinically and assess their symptoms (bone pain).
Tumor flare with HT has been reported in some patients with MBC with bone metastases during the first weeks of treatment with tamoxifen and toremifene (20). Tumor flare is a syndrome of diffuse musculoskeletal pain with increased size of tumor lesions rather than regression. It is often associated with hypercalcemia. Tumor flare does not imply failure of HT or represent tumor progression; rather, it suggests that the tumor will respond to HT. Symptoms should be treated while HT is continued. If hypercalcemia occurs, appropriate measures should be instituted (20).
SEQUENTIAL LINES OF HORMONAL THERAPY
It is important to remember that a patient with ER/PR+ MBC who responded well to first-line HT will likely respond to another line of HT, although the response rate and duration of response decrease.
When contemplating first or subsequent HTs in ER/PR+ MBC, our approach is to carefully assess patients’ symptoms and the tumor’s features such as degree of ER and/or PR expression, location of metastases (bone only vs. bone + limited visceral sites), disease burden (extent and number of metastases), and prior response to and length of benefit from HT. Asymptomatic patients, with low volume disease, even if visceral, but with high ER/PR expression and HER2− can be suitable candidates for further lines of HT.
 If there is no evidence of visceral crisis and there are available hormonal options, then we always consider another line of HT as the preferred systemic management.
If there is no evidence of visceral crisis and there are available hormonal options, then we always consider another line of HT as the preferred systemic management.
Postmenopausal Women
Postmenopausal women with ER/PR+, HER2− MBC have a variety of options for treatment. Therapies include AIs (anastrozole, letrozole, exemestane), SERMs (tamoxifen, toremifene), fulvestrant, and combination therapies with the CDK 4/6 inhibitor palbociclib and the mTOR inhibitor everolimus.
Selective Estrogen Receptor Modulators (tamoxifen, toremifene)
Tamoxifen (20 mg daily) is a nonsteroidal SERM with potent antiestrogenic properties, which are related to its ability to compete with estrogen for binding sites in target tissues. Tamoxifen is an established treatment option for ER/PR+ MBC in postmenopausal women. Tamoxifen is extensively metabolized after oral administration with N-desmethyl tamoxifen as the major metabolite found in plasma. Tamoxifen is a substrate of cytochrome P-450 3A, 2C9 and 2D6, and an inhibitor of P-glycoprotein (21). It is important to take into consideration drug interactions when prescribing.
Toremifene (60 mg daily) is another ER receptor agonist/antagonist indicated for the treatment of MBC in postmenopausal women with ER+ or unknown tumors. Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted to evaluate the efficacy of toremifene for the treatment of MBC in ER+ postmenopausal women. Two of the three studies showed similar results for all effectiveness end points, while the Nordic Study showed a longer time to progression (TTP) for tamoxifen (22). Based on these findings and the availability of newer hormonal agents and combinations, toremifene is rarely used in treatment of advanced ER/PR+ BC.
Aromatase Inhibitor (AI)
FIRST-LINE TREATMENT
AIs have compared favorably to tamoxifen with improvements in overall response rates (ORR), progression-free survival (PFS), and overall survival (OS). One of the largest phase III trials that included 916 women showed a significant benefit of first-line treatment with letrozole (2.5 mg daily) compared to tamoxifen in terms of PFS (9.4 vs. 6.0 months; HR 0.72; P < .0001) and RR (32% vs. 21%; P = .0002) with a nonsignificant increase in OS (23). An analysis of two phase III trials comparing anastrozole (1 mg daily) to tamoxifen showed a similar significant improvement in PFS (10.7 vs. 6.4 months; P = .022) in the ER/PR+ subgroup (24). Exemestane (25 mg daily) has been compared to tamoxifen in a nonblinded phase III trial of 371 women showing a similar significant improvement in RR (46% vs. 31%; P = .005) and PFS (9.9 vs. 5.8 months; P = .028 by Wilcoxon test) but no improvement in OS (25).
 A 2006 meta-analysis of 23 MBC trials with 8,504 women comparing AIs (first, second, and third generation agents) with tamoxifen showed a significant benefit in OS with the third generation AIs (HR 0.87, P ≤ .001) (26). The significant survival benefit was maintained in both first-line and second-line trials in this meta-analysis.
A 2006 meta-analysis of 23 MBC trials with 8,504 women comparing AIs (first, second, and third generation agents) with tamoxifen showed a significant benefit in OS with the third generation AIs (HR 0.87, P ≤ .001) (26). The significant survival benefit was maintained in both first-line and second-line trials in this meta-analysis.
Comparisons of AIs to one another have largely not shown differences in efficacy. Several studies compared exemestane to anastrozole, showing equal efficacy and no differences in ORR, PFS, or OS (27–29). Letrozole has been compared to anastrozole as second-line therapy in a phase III/IV trial with no difference in TTP, clinical benefit, or OS, albeit a significant improvement in ORR favoring letrozole was observed (19.1% vs. 12.3%; P = .013) (30). Consequently, no convincing data is available showing a preference of one AI over the other in the first- or second-line setting.
Fulvestrant is an SERD. It binds to the ER in a competitive mode with affinity comparable to estradiol and downregulates the ER protein in BC cells. It is Food and Drug Administration (FDA) approved for treatment of ER/PR+, MBC in postmenopausal women after disease progression on antiestrogen therapy, and for ER/PR+ HER2- advanced or MBC in combination with palbociclib after progression on endocrine therapy. Based on the FIRST trial, results that are discussed later, fulvestrant can also be considered for first-line treatment.
Fulvestrant dose is 500 mg intramuscularly on day 1, 15, 29, and then every 28 days. For patients with hepatic impairment (Child–Pugh class B), a dose of 250 mg is utilized but the same schedule is recommended.
FULVESTRANT VERSUS TAMOXIFEN
In an early first-line phase III trial of 587 women with ER/PR+ MBC, 250 mg of fulvestrant was compared to tamoxifen. There was no difference in PFS (6.8 vs. 8.3 months) or ORR (31.6% vs. 33.9%), respectively (31). These negative results were attributed to the lower dose of fulvestrant as subsequent prospective trials have shown a benefit to a higher 500 mg dose.
FULVESTRANT DOSAGE MATTERS
 The CONFIRM trial compared a 500 mg to 250 mg dose of fulvestrant in the second-line, AI refractory setting. A significant improvement in PFS was demonstrated with the higher dose which is now considered standard dose for treatment of MBC (32).
The CONFIRM trial compared a 500 mg to 250 mg dose of fulvestrant in the second-line, AI refractory setting. A significant improvement in PFS was demonstrated with the higher dose which is now considered standard dose for treatment of MBC (32).
FULVESTRANT AS FIRST-LINE THERAPY COMPARED TO AI
The FIRST trial compared fulvestrant to anastrozole as first-line therapy. It showed a significant increase in PFS favoring fulvestrant (23.4 vs. 13.1 months, HR 0.66; P = .01) (33) and clinical benefit rate (CBR) of 72.5% and 62%, respectively (34). A more recent update to the FIRST trial in 2015 suggests an OS benefit. Recently, the phase III FALCON trial was presented at the 2016 ESMO meeting; 462 patients (fulvestrant, n = 230; anastrozole, n = 232) were randomized. The primary endpoint was met showing statistically significant improvement in PFS with fulvestrant versus anastrozole (HR 0.797 [95% CI 0.637, 0.999]; P = .0486; median PFS, 16.6 versus 13.8 months, respectively) (35,36).
COMBINATION HORMONAL THERAPY WITH FULVESTRANT
The combination of fulvestrant and anastrozole has been compared to anastrozole alone in two trials with disparate results. Fulvestrant was given at 250 mg monthly, considered a nonstandard dose now. The FACT trial included 514 women and did not show any difference in PFS (10.8 vs. 10.2 months; HR 0.99; P = .91) (37). The SWOG 0226 trial, however, suggested the combination therapy was better in terms of PFS (15 vs. 13.5 months; HR 0.80; P = .007) and OS (47.7 vs. 41.3 months; HR 0.81; P = .049) (38). The FACT trial had more patients who received adjuvant chemotherapy and adjuvant tamoxifen therapy whereas the SWOG 0226 trial had more de novo metastatic disease.
Second-Line Therapy With Fulvestrant at 250 mg has been compared favorably to AIs, with no difference in OS (39). Fulvestrant has also been compared in combination with exemestane in the second-line setting in the SoFEA trial. No difference in PFS was found when the combination of fulvestrant and exemestane was compared to fulvestrant or exemestane alone (4.4 vs. 4.8 vs. 3.4 months, respectively; P = .98) (40).
MECHANISTIC TARGET OF RAPAMYCIN (mTOR) INHIBITORS IN MBC
The mTOR pathway mediates cell growth and metabolism and can be activated by a range of signaling factors such as ERs. Dysregulation of this pathway can lead to the resistance to endocrine therapy, whereby further targeted inhibition has been shown to restore endocrine sensitivity for clinical benefit (41). Everolimus 10 mg daily is FDA approved for treatment of ER/PR+ HER2− advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. Some of the most common adverse reactions with an incidence of ≥30% include stomatitis, infections, rash, fatigue, and diarrhea.
EVEROLIMUS AND EXEMESTANE
BOLERO-2 trial was a phase III trial that randomized 724 women who had progressed on AI therapy to combination therapy using exemestane + everolimus or exemestane + placebo. The combination treatment resulted in a significantly longer PFS (7.8 vs. 3.2 months, respectively; HR 0.45; P < .0001) and led to its FDA approval for use in this setting (42,43).
EVEROLIMUS AND TAMOXIFEN
TAMRAD trial was a phase II trial that assessed everolimus with tamoxifen compared to tamoxifen alone following progression on an AI. This showed superiority of the combination with a longer PFS (8.6 vs. 4.5 months; HR 0.54; P = .002) (44).
Toxicities notable in both mTOR studies included a higher rate of stomatitis, rash, fatigue, diarrhea, and anorexia (42–44).
TEMSIROLIMUS AND LETROZOLE
HORIZON trial was a phase III study that compared temsirolimus (an mTOR inhibitor) + letrozole to letrozole alone in the first-line setting. There was no benefit seen with the combination compared to letrozole alone (45).
The lack of benefit seen in HORIZON supports the restricted benefit of mTOR inhibition to those with acquired AI resistance.
TARGETED CYCLIN-DEPENDENT KINASE 4/6 INHIBITORS
Cyclin D1 and CDK4/6 are downstream of ER signaling pathways and activation of these pathways leads to cellular proliferation. In vitro, palbociclib reduces cellular proliferation of ER+ breast cancer cell lines by blocking progression of the cell cycle from G1 into S phase. CDK 4/6 inhibitors have recently been shown to be effective in the management of ER/PR+, HER2− MBC based on several clinical trials.
PALOMA-1 was a phase II study that showed that the combination of letrozole and palbociclib nearly doubled the PFS from 10.2 to 20.2 months compared to letrozole alone (HR 0.49; P = .0004) in the first-line setting in patients with ER/PR+ MBC (46). Notable toxicities in the experimental group were neutropenia, pulmonary embolism, diarrhea, and fatigue.
This led to the accelerated FDA approval of palbociclib in February 2015 contingent upon the confirmatory phase III PALOMA-2 trial.
PALOMA-3 was a phase III trial that compared palbociclib + fulvestrant to fulvestrant alone as second-line therapy following disease progression on prior endocrine therapy. It showed a similar doubling of PFS by 5.4 months (9.2 vs. 3.8 months, respectively; HR 0.42; P < .001) in the combination arm (47). This led to the FDA approval of palbociclib + fulvestrant in the second-line setting. What is not known, however, is the effectiveness of second- or third-line HT after palbociclib combinations.
PALOMA-2 was a phase III confirmatory trial of palbociclib + letrozole (P+LET) versus letrozole (LET) alone as first-line treatment of ER/PR+ MBC. The data from this trial was presented recently (48): the PFS was significantly longer for the combination of P+LET versus LET (HR 0.58, 95% CIs [0.46, 0.72]; P < .000001) and median PFS of 24.8 versus 14.5 mos, respectively.
Palbociclib for ER+ HER2+ disease is not currently approved for use given the lack of published clinical data; however, active clinical trials are under way. Preclinical data support that the combination may be effective (46).
Progestins
Megestrol acetate (MA) and medroxyprogesterone acetate are progestins with antiestrogenic properties that disrupt the ER cycle, possibly through inhibition of aromatase activity or action through the glucocorticoid receptor, androgen receptor, or progesterone receptor. Early randomized trials showed activity in ER/PR+ MBC with an approximate response rate of 25% and median duration of response of 15 months with activity seen following progression on tamoxifen (49,50).
CALGB 8741 was a phase III study that assessed dose escalation of MA in the treatment of ER/PR+ MBC. The response rates were 23%, 27%, and 27% for MA 160 or 800 or 1,600 mg/d, respectively, and no significant differences in the treatment arms were noted for TTP or for survival; survival medians were 28 months (low dose), 24 months (mid-dose), and 29 months (high dose) (49). Side effects were notable for increased thromboembolic events, weight gain, and fluid retention. These agents have been compared to AIs following progression on tamoxifen therapy in several studies with similar response rates and PFS but an improved side-effect profile favoring AIs (51–54).
A combined updated analysis of the two prior trials by Buzdar et al demonstrated a significant OS advantage favoring anastrozole versus MA (26.7 vs. 22.5 months; HR 0.78; P < .025) (55). Exemestane was compared to MA in a trial of 769 women demonstrating an improvement in PFS (4.7 vs. 3.8 months; P = .037; respectively) and OS (median not yet reached vs. 28.5 months; P = .039; respectively) (56).
Use of progestins in treatment of ER/PR+ MBC is sporadic given their toxicity profile and availability of more effective targeted agents and combinations.
Estrogen Therapy for ER/PR+ MBC
Although counterintuitive and seemingly paradoxical, estrogen has been used successfully in the treatment of ER/PR+ BC. The treatment efficacy of a synthetic estrogen, diethylstilbestrol (DES), in postmenopausal women with BC was noted in the 1940s, suggesting that low estrogen levels associated with the menopause may sensitize BC to DES. Some women treated with intermittent therapy had repeated regressions of disease upon reintroduction of DES. In the 1980s, tamoxifen was FDA approved and DES was eventually withdrawn from use in treatment of BC. Estradiol is still occasionally used in treatment of BC after the failure of newer endocrine therapies (56).
A randomized phase II trial in postmenopausal women with ER/PR+, AI-resistant MBC was done to compare clinical benefit of 30 mg estradiol daily (10 mg TID) with 6 mg daily (2 mg TID) and to determine if prior exposure to AI treatment sensitizes ER+ MBC to lower, better tolerated, and safer doses of estradiol. A total of 66 patients were treated and clinical benefit rates (CBRs) were 28% (30-mg arm) and 29% (6-mg arm) (57). The frequency of grade 3+ adverse events was higher in the 30-mg versus 6-mg arm (P = .03). Seven patients with estradiol-sensitive disease were retreated with an AI upon progression, two had partial responses and one stable disease suggesting resensitization to estrogen deprivation.
 Low-dose estrogen is rarely considered for treatment of ER/PR+ MBC. It is important to remember and to consider it only in settings when hormone resistance is established after multiple lines of antiestrogen therapies.
Low-dose estrogen is rarely considered for treatment of ER/PR+ MBC. It is important to remember and to consider it only in settings when hormone resistance is established after multiple lines of antiestrogen therapies.
Premenopausal Women With ER/PR+ MBC
In the following we discuss several effective treatment options for premenopausal women with ER/PR+ MBC including ovarian suppression/ablation, SERMS, combination therapy of ovarian suppression and SERM or AI, or SERD and other targeted agents.
Ovarian Suppression/Ablation
Ovarian suppression/ablation can be completed with luteinizing-hormone-releasing hormone (LHRH)/gonadotropin-releasing hormone (GnRH) agonists, radiotherapy to the ovaries, or oophorectomy. Ovarian suppression/ablation alone is effective therapy for premenopausal women with ER/PR+ MBC, with response rates ranging from 14% to 70% (58,59). In an early study of 136 premenopausal women randomly assigned to either ovarian suppression with goserelin or ovarian ablation with oophorectomy, no difference was seen in OS, failure-free survival, or RR (60). Ovarian suppression with hormone therapy in the advanced setting has been found to have increased PFS (HR 0.70; P = .0003) and OS (HR 0.78; P = .02) in a meta-analysis of GnRH alone or in combination with tamoxifen (61). Ovarian suppression induces a higher risk of osteoporosis, dyslipidemia, hot flashes, vaginal dryness, and mood swings, which are important factors to counsel the premenopausal population (61). Choice of therapy is largely based on patient and physician preference given the varied side-effect and long-term risk profiles.
For premenopausal women, we recommend ovarian suppression combined with a hormonal treatment that is the same as would be given for postmenopausal women (AI, SERD [62], targeted agent combinations) or treatment with a SERM alone for premenopausal women with MBC.
Selective Estrogen Receptor Modulators
SERMs such as tamoxifen have been used for many years for the treatment of MBC, although with limited data for the premenopausal population.
TAMOXIFEN
In prospective randomized studies comparing tamoxifen to ovarian ablation (oophorectomy or ovarian irradiation) in premenopausal women with MBC, the ORR, TTF, and OS were similar (63,64). Elevated serum and plasma estrogens have been observed in premenopausal women receiving tamoxifen, but the data from the randomized studies do not suggest an adverse effect of this increase. A limited number of premenopausal patients with progression of disease (PD) on tamoxifen responded to subsequent ovarian ablation.
Tamoxifen has shown to be equivalent to ovarian suppression in several trials conducted in premenopausal women with response rates of approximately 45% (18). A 1997 meta-analysis that evaluated 220 women showed no statistically significant difference in risk of disease progression or death (65).
Combination therapy of tamoxifen and ovarian suppression has shown an increased PFS (8.7 vs. 5.4 months; HR 0.70; P = .0003) and OS (2.9 vs. 2.5 years; HR 0.78; P = .02) when compared to ovarian suppression alone in a meta-analysis of four randomized trials (61). No trials have compared single agent tamoxifen with ovarian suppression and tamoxifen in MBC.
 Based on this data, our approach is to always utilize ovarian suppression in addition to tamoxifen in premenopausal women with ER/PR+ MBC.
Based on this data, our approach is to always utilize ovarian suppression in addition to tamoxifen in premenopausal women with ER/PR+ MBC.
Aromatase Inhibitors
AIs prevent the peripheral conversion of androgens to estrogens and are effective in many settings (chemoprevention, neoadjuvant, adjuvant, metastatic) for postmenopausal women. Their use is contraindicated in premenopausal women without the use of ovarian suppression due to the negative feedback loop from the pituitary, leading to an increase in estrogen production by the ovaries.
Limited phase II trials in premenopausal women have shown encouraging clinical benefit with ovarian suppression and AIs (66–69). It is important to remember that in the postmenopausal setting, AIs have showed superiority to tamoxifen as frontline therapy for ER/PR+ MBC, which has further supported their use in the premenopausal space, although direct evidence for this is lacking (26). Additionally, no trials have been published comparing ovarian suppression + AI to ovarian suppression alone; however, as mentioned previously, ovarian suppression and tamoxifen compared to ovarian suppression alone showed benefit for the combination with improved RR, PFS, and OS.
Cyclin-Dependent Kinase Inhibitor
The recently approved CDK 4/6-inhibitor, palbociclib, has shown clinical benefit in the large PALOMA-3 trial where palbociclib + fulvestrant was compared to fulvestrant alone as second-line therapy following progression of disease on prior endocrine therapy. This randomized, phase III trial showed a statistically significant prolongation of PFS by 5.4 mos (9.2 vs. 3.8 mos, respectively; HR 0.42; P < .001) in the combination arm (47). This trial included postmenopausal and premenopausal women on ovarian suppression comprising 20% of the study population. In subgroup analysis, premenopausal women had similar clinical benefit to postmenopausal women (HRs 0.44 and 0.41, respectively; P = .94) (47). This trial led to the recent expanded FDA approval for second-line therapy with fulvestrant and included pre/perimenopausal women in the indication.
PALOMA-1 was a phase II trial comparing letrozole alone to letrozole + palbociclib in postmenopausal women with ER/PR+ MBC as first-line therapy. PALOMA-1 showed benefit in terms of prolongation of PFS in the combination arm (46).
PALOMA-2 is a phase III study to confirm the phase II findings. Although premenopausal women on ovarian suppression were not included in either trial, we feel it is reasonable to consider ovarian suppression + letrozole + palbociclib in the first-line setting.
Selected Novel Agents in Clinical Trials for ER/PR+ MBC
PI3K INHIBITORS
Many exciting novel agents are in development for ER/PR+ MBC. Of the many novel agents, PI3K inhibitors such as buparlisib (BKM120) show promising results. The PI3K/AKT/mTOR pathway is implicated in many malignancies and in breast cancer PI3K mutations are common. The initial BELLE-2 (NCT01610284) results were presented at the 2015 San Antonio Breast Cancer Symposium. In this phase III randomized trial of fulvestrant versus fulvestrant + buparlisib, PFS was increased in the combination arm (6.9 vs. 5 months, HR .78; P < .001) (70). In evaluating the circulating tumor DNA, patients with mutant PIK3CA had an even greater benefit with combination therapy compared to fulvestrant alone (7 vs. 3.2 months, HR 0.56; P < .001). BELLE-3 (NCT01633060) is still recruiting and is evaluating buparlisib + fulvestrant after progression on an mTOR inhibitor. Additional PI3K inhibitors such as idelalisib, pictilisib, and alpelisib are being evaluated in clinical trials.
CDK INHIBITORS SELECTIVE FOR CDK4 AND CDK6
Abemaciclib has shown promising initial results gaining a FDA breakthrough designation. Abemaciclib shows initial single agent activity with an objective response rate of 33.3% and clinical benefit rate of 61.1% (71). A single-arm phase II trial of abemaciclib in previously treated patients (MONARCH-1-NCT02102490) and two phase III combination trials (MONARCH-2-NCT02107703 with fulvestrant and MONARCH-3-NCT02246621 with an AI) are ongoing. The results of the MONALEESA 2 trial (NCT01958021) were recently presented at the ESMO2016 meeting. In this trial, postmenopausal women (N = 668) with ER/PR+, HER2– MBC with no prior systemic treatment for MBC were randomized (1:1) to receive ribociclib (600 mg/day, 3-weeks-on/1-week-off) + letrozole (2.5 mg/day, continuous) or placebo + letrozole. The study met its primary objective: at the interim analysis, PFS was significantly improved in the ribociclib arm, with a HR of 0.556 (95% CI: 0.429–0.720; P = .00000329). Median PFS was not reached in the ribociclib arm (95% CI: 19.3–not estimable) versus 14.7 months in the placebo arm (95% CI: 13.0–16.5). In patients with measurable disease at baseline, ORR was 53% versus 37% (ribociclib vs. placebo arm; P = .00028) and CBR was 80% versus 72% (P = .02) (72).
HISTONE DEACETYLASE (HDAC) INHIBITOR
Entinostat is a novel class I HDAC inhibitor, which has been shown to inhibit growth factor signaling pathways that mediate hormone resistance. The ENCORE 301 trial compared entinostat + exemestane to exemestane + placebo in 130 heavily pretreated patients who were resistant to AIs. The PFS was 4.28 versus 2.27 months (HR 0.73; P = .055) with an exploratory end point showing benefit in median OS, 28.1 versus 19.8 months (HR 0.59; P = .036) (73). A phase III trial E2112 (NCT02115282) with entinostat + exemestane versus exemestane + placebo in ER/PR+, HER2−, pre and post menopausal women and men who failed an AI therapy in first line setting is ongoing.
![]() MANAGEMENT PEARLS—MBC HORMONAL THERAPY
MANAGEMENT PEARLS—MBC HORMONAL THERAPY
1. The presence and degree of tissue ER/PR expression strongly predicts response to hormonal treatments, with responses seen in approximately 60% of women with both ER+ and PR+ tumors, versus 30% in women with either ER+ or PR+ status alone, versus less than 10% of women with receptor negative (ER-/PR-) disease. Patients with both ER+ and PR+ tumors have a more favorable prognosis with longer OS than their counterpart single hormone receptor positive tumors.
2. HT should be continued until there is clear evidence of disease progression. Some patients with ER/PR+ MBC may experience prolonged periods of disease stability (years) while HT is continued and quality of life is preserved.
3. We do not recommend combining HT agents with chemotherapy in ER/PR+ MBC.
4. It is important to remember that a patient with ER/PR+ MBC who responded well to first-line HT may respond to another line of HT, although the response rate and duration of response decrease.
5. Not all visceral disease requires upfront chemotherapy. If there is no evidence of visceral crisis and there are available hormonal options, we always consider another line of HT as the preferred systemic management.
CHEMOTHERAPY
Single Agent Versus Combination Chemotherapy
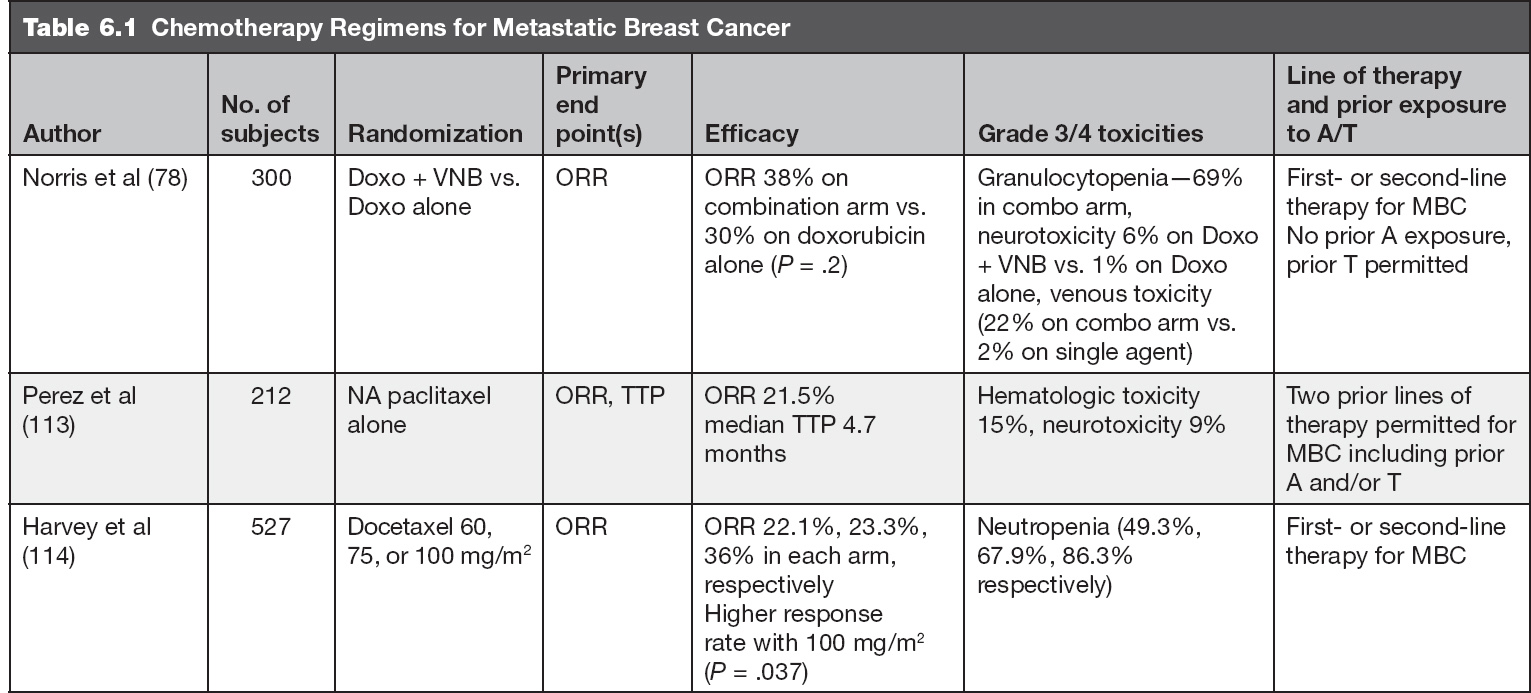
There are no prospective data that demonstrate conclusively that combination chemotherapy improves survival when compared to single agent cytotoxic chemotherapy for treatment of MBC. However, combination chemotherapy has been shown to increase overall response rates in MBC. Therefore, when a higher response rate is vital, such as in patients with visceral crisis, combination chemotherapy may be appropriate. Otherwise, single agent therapy is preferred to avoid cross-resistance to multiple agents and to limit toxicities.
In a prospective randomized study comparing first-line single agent epirubicin (E) to combination cyclophosphamide, epirubicin, and fluorouracil (ECF), no significant difference in survival or TTP was appreciated between the two treatments (74). Similar results were shown comparing sequential versus concomitant administration of an anthracycline and taxane as first-line treatment of MBC (75). ECOG 1193 did show that combination of doxorubicin and paclitaxel (AT) was associated with a higher ORR and longer median TTP than sequential therapy as first-line therapy for MBC, but with greater toxicity and no difference in OS (76).
The European MBC task force performed a literature review of different monotherapy versus combination therapy comparison studies. None of these studies showed meaningful differences in PFS or OS (77).
Single Agent Chemotherapy
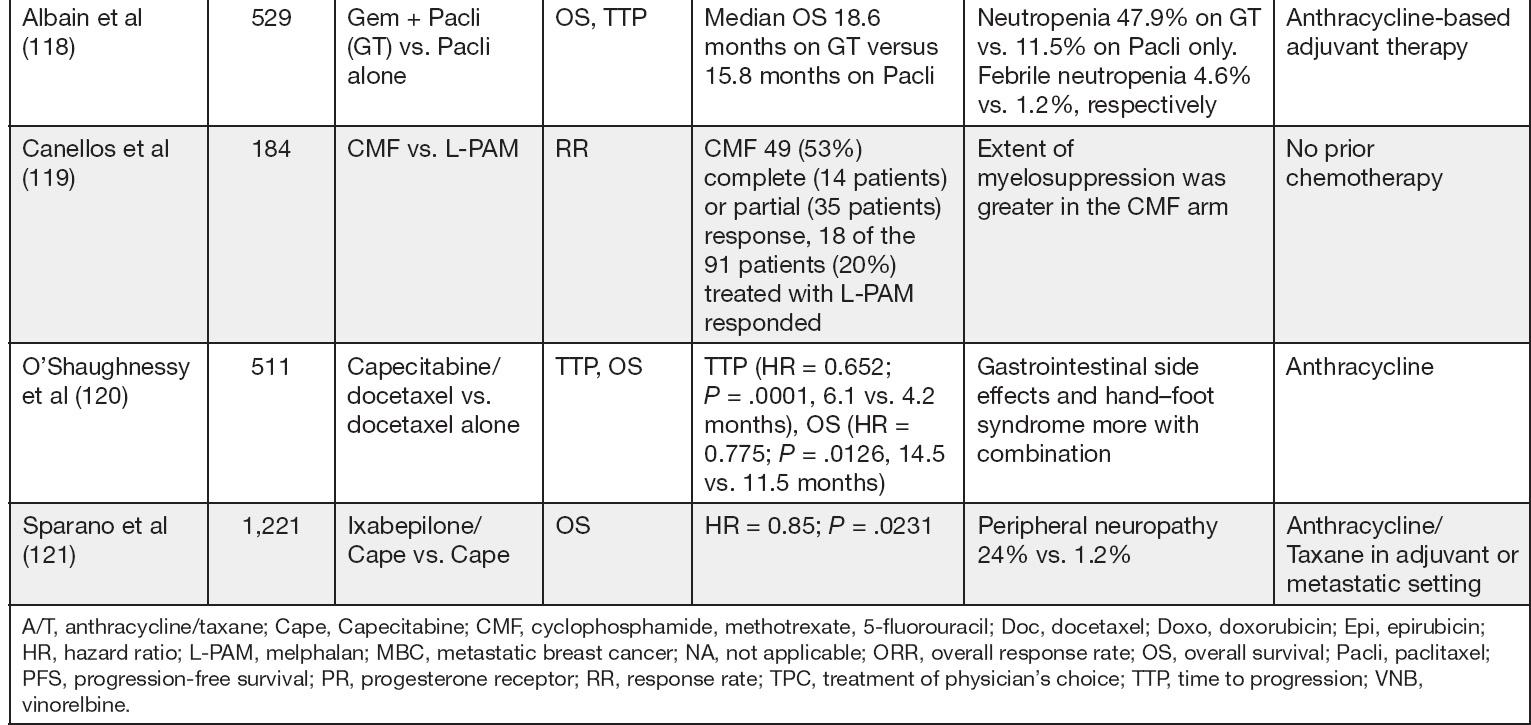
Anthracyclines are active agents in the treatment of MBC. Cumulative cardiac toxicity inherent to prolonged use becomes a concern in the metastatic setting and limits its use in treatment of advanced breast cancer due to the frequent utilization of these agents in the adjuvant setting. However, in the setting of de novo presentation of advanced breast cancer, anthracycline as single agent or in combination (AC, EC, or TAC) can be considered when the visceral burden of disease is high and indications for chemotherapy are present (see p. 175).
DOXORUBICIN
In a phase III study, doxorubicin alone was compared to combination therapy with vinorelbine, and the treatments were found to be similar with respect to TTP and OS (78). The dosing schedule for doxorubicin alone is 60 to 75 mg/m2 every 3 weeks or 20 mg/m2 weekly.
Epirubicin is a structural analog of doxorubicin with similar efficacy, but with some data to suggest a lower rate of cardiac toxicity (79). The half-life of epirubicin is much shorter than doxorubicin but the peak plasma concentration is similar. A prospective comparison study of epirubicin (85 mg/m2) versus doxorubicin (60 mg/m2)every 21 days in patients with advanced BC who failed nonanthracycline combination chemotherapy showed that ORR = 25% in both arms; median duration of response was 11.9 mos for epirubicin and 7.1 mos for doxorubicin. The median doses to the development of laboratory cardiotoxicity were estimated to be 935 mg/m2 (epirubicin) and 468 mg/m2 (doxorubicin) and the median cumulative dose at which congestive heart failure (CHF) was observed was 1,134 mg/m2 (epirubicin) versus 492 mg/m2 (doxorubicin) (79). Epirubicin just like doxorubicin can be dosed at 20 to 25 mg/m2 weekly.
Pegylated liposomal doxorubicin (PLD) was developed to further improve the safety profile of anthracyclines. PLD is doxorubicin confined within liposomes stabilized by grafting polyethylene glycol onto its surface. PLD has a longer half-life (55 hours) prolonging the circulation time (80). In a study comparing PLD to doxorubicin, median PFS and OS were comparable in the two arms (80,81). PLD was associated with a decreased risk of cardiomyopathy, even in high-risk patients, although with a higher incidence of dose-related palmar plantar erythrodysesthesia (PPE) (80,81). PLD is commonly given at a dose of 40 to 50 mg/m2 every 3 to 4 weeks.
TAXANES
Taxane-containing regimens improve OS, TTP, and tumor ORR in women with MBC and are routinely prescribed as first- and later-line therapy in the metastatic setting due to high ORR noted in multiple studies (82–84). In a Cochrane review, taxanes were found to be superior to anthracycline and other nonanthracycline regimens in terms of PFS and OS (85). The combined HR for OS and TTP favored the taxane-containing regimens (HR 0.93; P = .002; 0.92; P = .002) respectively. For studies of first-line chemotherapy, this effect persisted for OS (HR 0.93; P = .03) but not for TTP (HR 0.96; P = .22). Response rates were also better with taxane-containing chemotherapy (HR 1.20; P < .00001).
Paclitaxel was initially given as a 250 mg/m2 96-hour infusion and demonstrated a response rate of 56% as initial chemotherapy (82). Further studies utilized shorter infusions of paclitaxel (175 mg/m2 by 3-hour infusion every 21 days) in patients previously treated with the FEC as an adjuvant or first-line therapy for MBC and was associated with ORR of 54% for adjuvant FEC and 60% for 5-flurouracil/epirubicin/cyclophosphamide (FEC) as first-line treatment (86). Other phase II studies of paclitaxel have shown comparable results (83,84). Paclitaxel given weekly (dose range 80–108 mg/m2) was associated with the ORR of 53%, with 10% complete response (CR) (87). Median response duration was 7.5 months (range, 2 to 11+). Responses were observed in 50% of patients who had received prior anthracycline therapy, including in half of patients with disease progression on anthracycline within 1 year (87).
Paclitaxel is most commonly given on a weekly schedule at 80 mg/m2, based on a 2010 meta-analysis that showed improved OS and improved tolerability over an every 3-week schedule (88).
Docetaxel differs from paclitaxel in its pharmacokinetic profile. The cellular uptake is higher with docetaxel compared to paclitaxel, and the efflux rate is about thrice slower for docetaxel. This contributes to the greater cytotoxicity of docetaxel than paclitaxel. Data suggests there is only partial cross-resistance between paclitaxel and docetaxel. A phase II study that evaluated the response to docetaxel (75–100 mg/m2 every 21 days) in patients with paclitaxel-resistant MBC showed an ORR of 17.4% (89). Weekly docetaxel at 40 mg/m2 demonstrated an ORR of 41% in the metastatic setting in a phase II study (90). However, it is more commonly given on an every 3-week schedule, based on a study in the adjuvant setting, which demonstrated an improvement in DFS with this schedule (88).
When comparing these two taxanes, toxicities do differ. Generally paclitaxel results in more neuropathy and myalgia, whereas docetaxel generally causes more febrile neutropenia, edema (which can be ameliorated with the use of dexamethasone), and gastrointestinal side effects (diarrhea).
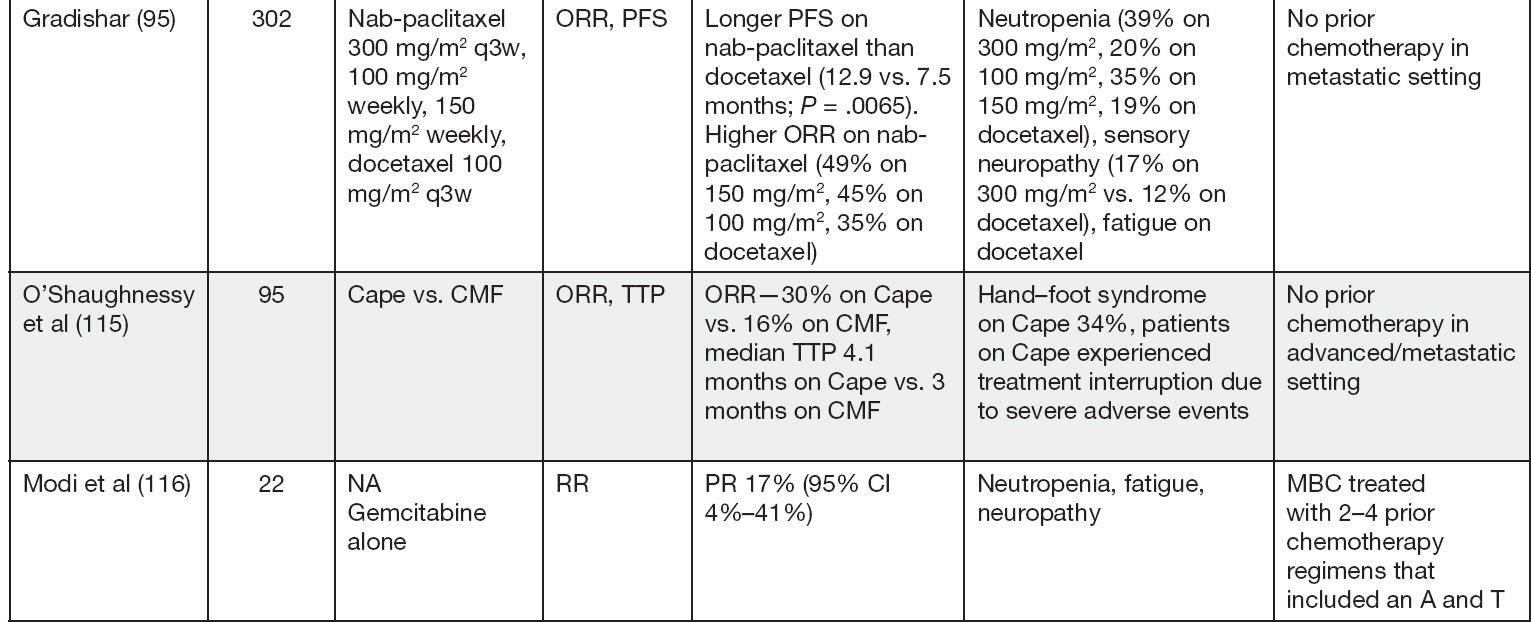
Nab-paclitaxel (albumin-bound solvent-free paclitaxel) was designed to overcome the infusion reactions experienced in relation to the solvents used in paclitaxel. These solvent vehicles were also thought to impair drug delivery to the tumor and be responsible for the disproportionate systemic drug exposure (91). Nab-paclitaxel and paclitaxel have been compared in xenograft tumor models at equitoxic doses, and nab-paclitaxel was found to have greater antitumor activity (92). When compared to docetaxel, nab-paclitaxel resulted in significantly longer PFS in the first-line setting (93–96). Nab-paclitaxel may be dosed at 100 to 150 mg/m2 on days 1, 8, and 15 of a 28-day cycle or 260 mg/m2 every 3 weeks (94–96).
OTHER AGENTS
Capecitabine is an oral prodrug and is converted to its active form 5-fluorouracil by the enzyme pyrimidine nucleoside phosphorylase. This enzyme is expressed at high levels by various tumor, which results in precise delivery of active drug to the tumor tissue and reduces bowel exposure to the active drug. A patient with MBC who does not want intravenous (IV) chemotherapy may choose to start with the oral capecitabine. The FDA-approved capecitabine at (1,250 mg/m2 twice daily orally for 2 weeks on and 1 week off) but this dose is often difficult to tolerate and patients often require dose reductions. Many oncologists start with alternative dosing schedules and use 1,000 mg/m2 bid × 14 days, 7 days off schedule. Studies have shown these dose reductions do not impair efficacy of the drug (97). In a phase II study of patients with paclitaxel-refractory disease, ORR was approximately 20%. The most common adverse events reported include hand–foot syndrome, diarrhea, nausea, and vomiting. The median TTP was 93 days, and median duration of response was 8.1 months (96). In a first-line study, ORR was 26.1% with a dose of 2,000 mg/m2 (98). In another first-line phase II randomized study, ORR was 30% with capecitabine compared to 16% with Cytoxan, methotrexate, and 5-fluorouracil (CMF). Median TTP was longer with capecitabine versus CMF (4.1 vs. 3 months) respectively but survival was similar in the two treatment arms (99).
Gemcitabine is a nucleoside analog that has shown significant antitumor activity across a wide range of tumors with low systemic toxicity. It is generally administered at a dose of 1,000 mg/m2 weekly for 2 weeks followed by a 1-week rest. In the first-line setting, a response rate of 37% was noted (100), and in the second-line, 26% (101). When given as third- or fourth-line treatment in MBC, it has an ORR of 17% to 19% (102).
Eribulin mesylate is a nontaxane microtubule inhibitor; it is active in cell lines that have become resistant to taxanes. It is generally administered at a dose of 1.4 mg/m2 on day 1 and 8 of a 21-day cycle. In a phase II study, eribulin demonstrated an objective RR of 11.5% (103). In a phase III open-label randomized study, eribulin was compared to various therapies in heavily pretreated MBC (EMBRACE trial). The control arm consisted of treatment of physician’s choice (TPC). There was a significant increase in OS for the eribulin group compared to the TPC group (13.1 months vs. 10.6 months). More common grade 3 or 4 adverse events that occurred with eribulin were neutropenia, leukopenia, and peripheral neuropathy (104).
Vinorelbine is a semisynthetic vinca alkaloid that inhibits microtubule assembly and interferes with formation of the mitotic spindle and prevents cell division. It is generally administered at a dose of 25–30 mg/m2 weekly on days 1 and 8 in a 3-week cycle. As first-line treatment in advanced breast cancer, objective ORR was shown to be 35% (105,106,107,108). It has an objective ORR of 47% as second- or third-line treatment in MBC and no cross-resistance was documented with prior anthracycline or taxane treatment (105). The mechanism of action of docetaxel and vinorelbine has been found to be synergistic in preclinical models. In a phase II study of first-line therapy for patients who were taxane-naïve, the combination of docetaxel and vinorelbine resulted in an ORR of 59% (109).
Ixabepilone is an epothilone B analog, nontaxane microtubule-stabilizing compound, that has activity in taxane-resistant patients. This drug was evaluated in a phase II study of patients who were resistant to anthracyclines, taxanes, and capecitabine. ORR was 18.3% in this heavily pretreated population and a manageable toxicity profile was noted (110). A randomized phase III trial in patients with locally advanced MBC with prior anthracycline and taxane exposure with ixabepilone (40 mg/m2 IV q 3 weeks) and capecitabine (2,000 mg/m2/d days 1–14) versus capecitabine (2,500 mg/m2/d days 1–14) was done. Ixabepilone + capecitabine prolonged PFS compared to capecitabine alone (median, 5.8 vs. 4.2 mos), with a 25% reduction in the estimated risk of disease progression (HR, 0.75; P = .0003). Objective response rate was also increased (35% vs. 14%; P < .0001) (111, 208). The usual dose of Ixabepilone is 40 mg/m2 IV every 21 days.
Platinum agents including cisplatin and carboplatin are usually administered as part of combination regimens as there are limited single agent responses noted with these drugs. This class of drugs may be particularly useful in patients who have tumors in which DNA damage repair pathways are impaired, such as with BRCA mutations. Platinum agents (carboplatin or cisplatin) are typically combined with paclitaxel or gemcitabine in MBC, standard doses of these agents are used.
MAINTENANCE CHEMOTHERAPY
A systematic meta-analysis of 11 randomized chemotherapy clinical trials for MBC including 2,269 patients showed that longer first-line chemotherapy duration resulted in a marginal improvement in OS (HR, 0.91, 95% CIs [0.84, 0.99]; P = .046) and a substantial improvement in PFS (HR, 0.64, 95% CIs [0.55, 0.76]; P < .001) (112). There were no differences in effect on either OS or PFS between subgroups defined by time of random assignment, study design, number of chemotherapy cycles in the control arm, or concomitant endocrine therapy. The number of cycles of chemotherapy in the standard duration groups (control arms) ranged from 3 to 8 while the number of maintenance chemotherapy cycles ranged from 6 to continuing until disease progression or unacceptable toxicity. In terms of clinical benefit on OS, the 9% reduction in the hazard of death for longer duration of chemotherapy was felt to be marginally beneficial. In practice, this needs to be considered against toxicity.
![]() MANAGEMENT PEARLS—CHEMOTHERAPY (Table 6.1)
MANAGEMENT PEARLS—CHEMOTHERAPY (Table 6.1)
1. There are no prospective data that demonstrate conclusively that combination cytotoxic chemotherapy meaningfully improves survival when compared to single agent sequential chemotherapy for treatment of MBC. However, combination chemotherapy has been shown to increase ORR.
2. When a higher response rate is vital, such as in patients with visceral crisis, combination chemotherapy may be appropriate. Otherwise, single agent therapy is preferred to avoid cross-resistance to multiple agents and to limit toxicities.
3. Maintenance chemotherapy: A systematic meta-analysis of 11 randomized chemotherapy clinical trials in MBC including 2,269 patients showed that longer first-line chemotherapy duration resulted in a marginal improvement in OS (HR, 0.91, 95% CIs [0.84, 0.99]; P = .046) and a substantial improvement in PFS. This marginal benefit has to be weighed against increased toxicities of maintenance cytotoxic chemotherapy.
HER2+ MBC
HER2 positivity, seen in approximately 20% of breast cancers, is a prognostic and predictive marker in MBC (122). Historically, tumor tissue overexpression of HER2 was associated with increased risk of recurrence and worse overall prognosis. Since the introduction of HER2 targeted agents, the disease course in metastatic setting has been altered with improvement in median survival now reaching 56 months as seen in the CLEOPATRA trial (123).
Anti-HER2 therapy indications follow the previously described immunohistochemistry and in situ hybridization guidelines as set by the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) and previously described in Chapter 5. HER2 therapy is typically administered in combination with hormone and chemotherapy as described in the following.
Triple-Positive ER/PR/HER2+ MBC
Nearly 50% of all HER2-positive breast cancers will also coexpress ER or PR receptors (124,125). Triple-positive MBC can be treated with HER2 directed therapy, chemotherapy, and/or hormonal therapy (HT). Here we also do not recommend concurrent chemotherapy with HT and recommend sequential approaches using guidelines discussed in this chapter.
Society guidelines differ in the treatment algorithm for hormone positive HER2+ given the lack of evidence in randomized controlled trials.
• ASCO guidelines support recommendations for the upfront treatment in triple-positive MBC with differing “strengths” based on available evidence (126).
![]() HER2 targeted therapy combined with chemotherapy is a “strong” recommendation.
HER2 targeted therapy combined with chemotherapy is a “strong” recommendation.
![]() HER2 targeted therapy combined with endocrine therapy is a “moderate” recommendation.
HER2 targeted therapy combined with endocrine therapy is a “moderate” recommendation.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


