Management of Ductal Carcinoma In Situ | 4 |
Susan B. Kesmodel, Natalie O’Neill, Steven J. Feigenberg, Alex Engelman, Katherine H. R. Tkaczuk, Susan Shyu, and Olga Ioffe
INTRODUCTION
Ductal carcinoma in situ (DCIS) of the breast is a neoplastic lesion confined to the breast ducts and lobules without evidence of invasion into the surrounding stroma by light microscopic examination. Pure DCIS is considered a localized disease and is not associated with metastases to nodal basins (axillary, internal mammary, or supraclavicular lymph nodes) or distant metastases. The risk of metastases or death in a patient with pure DCIS is extremely low (less than 1%).
Epidemiology
The incidence of DCIS has significantly increased in the United States, from 1.87 per 100,000 women in 1973–1975 to 32.5 per 100,000 women in 2004. The incidence increased in all age groups but in particular for women older than 50 years (1). About 25% of breast cancers in the United States are DCIS and approximately 65,000 new cases of DCIS will be diagnosed in the United States alone in 2016 (Surveillance, Epidemiology, and End Results [SEER]).
Risk Factors
Similar to invasive breast cancer, risks for developing DCIS include older age, increased breast density, nulliparity or late age at first live birth, obesity, and family history.
Clinical Presentation
The majority of patients with DCIS have no clinical symptoms and are diagnosed via screening mammography. However, abnormal nipple discharge, a palpable mass, or Paget disease of the nipple can be associated with DCIS. Microcalcifications (Figure 4.1) seen on mammogram are very commonly associated with DCIS (90%). Certain mammographic patterns are highly suggestive of DCIS, such as linear branching or segmental types of pleomorphic microcalcifications (Chapter 1).
Diagnostic Evaluation
An abnormal lesion detected by mammogram or breast MRI should be assessed by core tissue sampling to obtain tissue confirmation of pathologic diagnosis. Excisional or incisional biopsy is rarely needed for diagnosis of DCIS and we typically do not recommend it unless the patient cannot have an image-guided biopsy. Fine needle aspiration (FNA) is not recommended because it may not provide enough tissue to confirm the diagnosis of noninvasive versus invasive breast carcinoma.
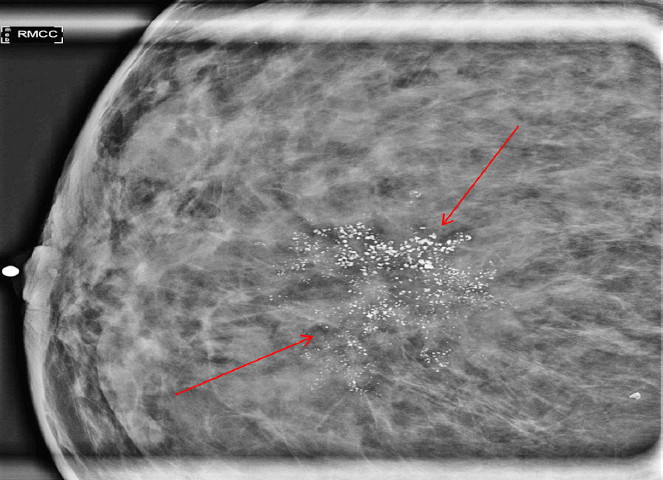
Figure 4.1 Mammographic magnification view of calcifications detected on screening mammogram; suspicious calcifications with pleomorphic morphology and grouped distribution (arrows) are noted; pathology: ductal carcinoma in situ (DCIS).
Pathology
DCIS is a neoplastic intraductal lesion characterized by the clonal proliferation of malignant epithelial cells, which, unlike invasive carcinoma, remain limited to the basement membrane of the ducts. DCIS is considered a direct precursor to invasive breast cancer and patients with DCIS have a risk 8 to 11 times greater than that of the general population for developing invasive carcinoma.
Grading
There is currently no universal agreement on a grading system for DCIS. Recently, there has been a shift toward nuclear grade and the presence of necrosis, as these factors are predictive of clinical outcome. The grade of DCIS is divided into three tiers: low, intermediate, and high. The characteristics of DCIS documented in the surgical pathology report are: (a) nuclear grade (based on nuclear atypia), (b) necrosis, and (c) architectural pattern.
Other key associated features noted on the pathology report include margin status, size of the lesion, and the presence of microcalcifications.
It is important to note that the tiered grading system does not necessarily imply a pathophysiologic progression from low- to high-grade DCIS. The current proposed sequence for the development of breast cancer involves two distinct pathways. Low/intermediate-grade DCIS is part of the low-grade/estrogen receptor-(ER) positive pathway thought to arise from the ER-expressing luminal cells and proliferative precursor lesions such as atypical ductal hyperplasia (ADH) before progressing to low-grade invasive carcinoma. In contrast, high-grade DCIS is considered part of the high-grade/ER-negative pathway with an unknown precursor that progresses to high-grade invasive breast cancer.
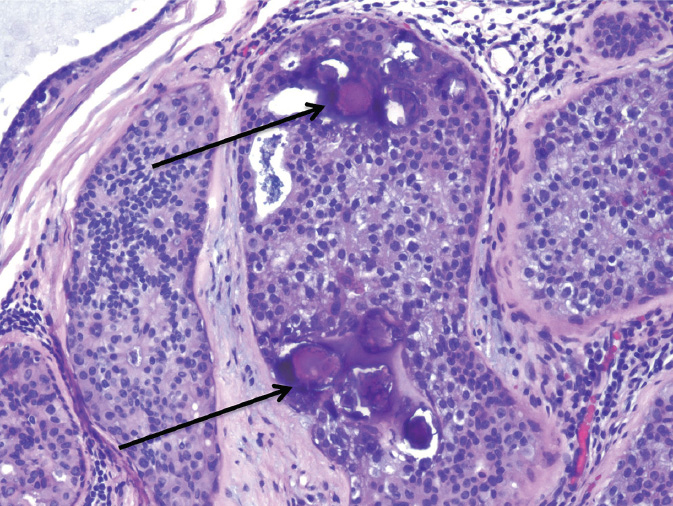
Figure 4.2 Low-grade ductal carcinoma in situ (DCIS), cribriform type, with calcifications (arrows).
Low-grade DCIS consists of small, round, monotonous cells, typically growing in a cribriform, micropapillary, or solid pattern (Figure 4.2). In the cribriform pattern, the cells are polarized around punched out (“cookie cutter”) luminal spaces evenly distributed throughout the lesion. The cells within the intervening strands or bridges are arranged regularly or lie at a right angle (“Roman arches”). In the micropapillary pattern, cells protrude into the lumens as club-like fronds or pseudopapillae lacking fibrovascular cores. True papillary growth pattern with fibrovascular cores may occur, as well as free floating clusters of polarized cells detached from the papillae. In the solid pattern, the involved duct is distended by solid growth of the proliferating neoplastic cells. Calcifications are frequent. Limited areas of central necrosis may be present.
Intermediate DCIS consists of cells with features intermediate between those of low-grade and high-grade DCIS. Calcifications are often present. Necrosis may or may not be seen. Of the three grades, intermediate DCIS has been shown to have the least interobserver reproducibility.
High-grade DCIS consists of cells with overt morphologic features of malignancy (Figure 4.3). There is marked nuclear pleomorphism and high nuclear grade with prominent nucleoli. Polarization is lost and mitotic figures are numerous. Extensive necrosis is common; comedo-type necrosis—necrotic debris in a duct lumen surrounded by solid growth of viable tumor cells—is considered a defining feature of comedo DCIS, a subtype of high-grade DCIS commonly associated with a mass and most likely to have an associated invasive carcinoma.
Paget disease of the nipple is caused by high-grade DCIS involving the underlying subareolar ducts; these malignant cells creep up into the nipple skin and undermine it, causing ulceration. The malignant cells in the epidermis, known as Paget cells, are large with abundant pale cytoplasm often containing mucin, pleomorphic nuclei, and prominent nucleoli.
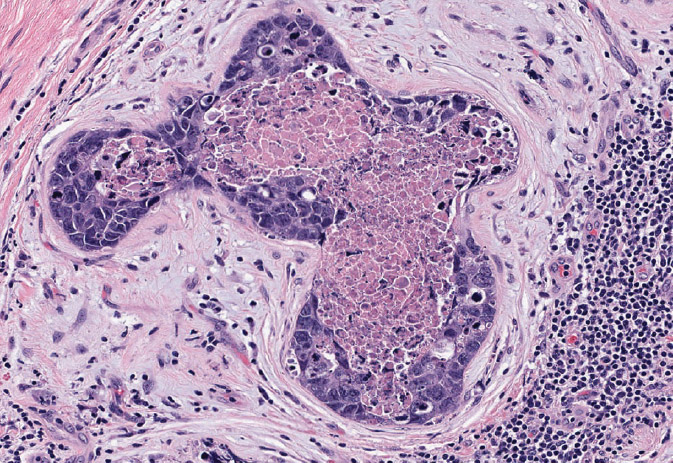
Figure 4.3 High-grade solid DCIS with central comedo-type necrosis (comedocarcinoma). The neoplastic cells are highly atypical, with frequent mitoses. Note stromal reaction and inflammatory infiltrate around DCIS.
Unusual variants include apocrine, neuroendocrine, mucinous and signet ring cell DCIS, and DCIS with basal-like phenotype (ER/PR/HER2-neu “triple negative”). The same assessment of nuclear grade and necrosis as that of the more usual DCIS applies to these variants.
Hormone receptor expression: The majority of DCIS, particularly low or intermediate grade, express receptors for estrogen (ER) and progesterone (progesterone receptor [PR]). The American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines recommend classifying all cases with <1% positive cells as receptor negative. For DCIS with >1% positive cells, the percentage of positive cells is reported along with the intensity of staining.
SURGICAL MANAGEMENT OF DUCTAL CARCINOMA IN SITU
The surgical management of DCIS is similar to that of early-stage invasive breast cancer.
Management of the Breast
Although there are no randomized controlled trials (RCTs) that have compared mastectomy to breast conserving surgery (BCS) in patients with DCIS, based on the results of clinical trials for early-stage, invasive breast cancer, both of these surgical approaches are accepted for DCIS (see Surgery, Early-Stage Invasive Breast Cancer, Chapter 5).
Breast Conserving Surgery
The use of BCS for DCIS has increased steadily. A review of patients treated from 1992 to 1999 demonstrated an increase in the use of BCS over time, and overall during this time period 64% of patients were treated with BCS (2). A more recent analysis that examined patients treated at different time points from 1991 to 2005 also demonstrated an increase in the use of BCS for DCIS in more recent years (3).
Patients with biopsy-proven DCIS are considered candidates for BCS if the area of involvement can be removed with negative margins and results in acceptable cosmesis. BCS is not recommended for patients with DCIS who have diffuse malignant appearing or indeterminate calcifications in the breast.
PROCEDURE
BCS refers to removal of the area of malignancy in the breast with a margin of normal surrounding breast tissue.
Since DCIS is usually a nonpalpable lesion, preoperative wire-guided localization via either ultrasound or stereotactic guidance is generally utilized to localize the area of abnormality in the breast. Larger areas of calcifications in the breast may be bracketed with two or more wires. The area can then be targeted by the surgeon for removal. A specimen radiograph is typically obtained in the operating room to confirm that the appropriate tissue has been removed with a margin.
Shave margins are additional margins that may be taken after a lumpectomy is performed. These margins may encompass the entire surgical cavity in all directions or may be selectively taken in certain directions based on gross examination of the lumpectomy specimen or review of the specimen radiograph. These margins provide pathologists with additional tissue for examination and have been shown to decrease the rate of positive margins.
OUTCOMES
 Long-term survival for patients with DCIS is excellent whether BCS or mastectomy is utilized for treatment.
Long-term survival for patients with DCIS is excellent whether BCS or mastectomy is utilized for treatment.
Adjuvant radiation therapy (RT) and endocrine therapy after BCS for DCIS have both been shown to decrease the risk of ipsilateral (invasive and noninvasive) breast tumor recurrence (IBTR).
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17 trial randomized patients with localized DCIS to lumpectomy alone versus lumpectomy with adjuvant RT. Eight-year follow-up data from this trial showed that for those patients who received adjuvant external radiation therapy (XRT), the incidence of noninvasive IBTR was reduced from 13.4% to 8.2% (P = .007), and invasive IBTR was reduced from 13.4% to 3.9% (P < .0001) (4). A 15-year follow-up evaluation of results from the NSABP B-17 trial and a second trial, NSABP B-24, which randomized patients with localized DCIS to lumpectomy and adjuvant XRT with or without adjuvant tamoxifen, showed that invasive IBTR was lowest in patients who received adjuvant XRT and tamoxifen after lumpectomy. The overall prognosis was excellent for patients with DCIS regardless of treatment, with breast-cancer-related deaths of <5% at 15 years for all treatment groups (5).
MARGINS FOR BCS IN PATIENTS WITH DCIS
 Studies are inconsistent in their definition of negative margins following BCS for patients with DCIS; however, based on recent studies, a margin of at least 2 mm is recommended.
Studies are inconsistent in their definition of negative margins following BCS for patients with DCIS; however, based on recent studies, a margin of at least 2 mm is recommended.
A retrospective, single-institution analysis of 469 patients treated for DCIS with BCS with or without radiation demonstrated that the likelihood of local recurrence (LR) was significantly greater for patients with a margin of <1 mm and that these patients benefitted the most from adjuvant RT. In patients with margins of <1 mm who did not receive adjuvant RT, the relative risk (RR) of recurrence was 2.54 compared to patients who received adjuvant RT (6). Similar results were reported from an analysis of patients treated on the European Organisation for Research and Treatment of Cancer (EORTC) 10853 trial, which found a hazard ratio (HR) of 2.07 for recurrence in patients with involved or close margins <1 mm compared to negative margins (7).
A recent meta-analysis of 22 trials that examined risk of recurrence in patients with DCIS treated with BCS and adjuvant RT showed that risk of recurrence was lower with negative margins compared to close/positive margins. When specific margin widths were compared, there was a significant difference in risk of recurrence when comparing margins of at least 5 mm to no tumor on ink or <1-mm margins. However, when margins of at least 5 mm were compared to 2-mm margins, there was no significant difference in LR rates (OR = 1.51; 95% CIs [0.51, 5.04]; P > .05). In addition, when a specific threshold margin was examined, a 2-mm margin was found to be superior to a margin <2 mm (OR = 0.53; 95% CIs [0.26, 0.96]; P < .05) (8).
 Based on these studies, we typically recommend obtaining a margin of at least 2 mm in patients with pure DCIS.
Based on these studies, we typically recommend obtaining a margin of at least 2 mm in patients with pure DCIS.
Mastectomy
Mastectomy may be considered in all patients with a diagnosis of DCIS. However, it is usually recommended for patients with a large area of involvement of the breast that does not appear to be amenable to BCS. This includes patients with diffuse malignant-appearing or indeterminate calcifications in the breast, a large area of involvement in the breast relative to breast size, and persistently positive margins after multiple excisions. It may also be recommended in patients who are not candidates for adjuvant RT.
Outcomes
A large meta-analysis of long-term outcomes for treatment of DCIS with mastectomy estimated an LR rate of 2.6% and breast-cancer-related death rate of 2% at 10 years (9).
SKIN-SPARING MASTECTOMY
Skin-sparing mastectomy (SSM) is a procedure that removes all breast tissue including the nipple–areolar complex (NAC) while preserving the skin envelope of the breast. This improves the cosmetic results from reconstruction. SSM is generally performed in patients with early-stage breast cancer including DCIS.
Outcomes
A retrospective, single-institution review of 223 patients with DCIS treated with SSM and immediate reconstruction with a median follow-up of 82.3 months showed an LR rate of 3.3% (10). In another study that included patients with invasive and noninvasive breast cancer undergoing SSM, 54 patients with DCIS were evaluated. The median follow-up time for all patients in the study was 119 months, with an LR rate of 2% in those patients with DCIS (11). Therefore, SSM is an oncologically safe procedure in appropriately, selected patients with DCIS. This approach may not be possible in patients with diffuse involvement of the breast tissue due to difficulty obtaining adequate margins.
NIPPLE-SPARING MASTECTOMY
Nipple-sparing mastectomy (NSM) removes all breast tissue and preserves the entire skin envelope of the breast including the NAC. This procedure may be considered in patients with DCIS when the DCIS does not involve the NAC. Typically, it is recommended that the tumor be at least 1 to 2 cm away from the NAC.
Outcomes
In a small review that examined NSM in 51 patients with DCIS in which 19 patients were followed for recurrence, an LR rate of 5.3%, 1/19 patients, was observed (12). A similar study that examined LR rates in patients undergoing NSM who were treated at the University of California San Francisco and Duke University and included 111 patients with DCIS showed an LR rate alone of 1.8%, 2/111 patients, and simultaneous local and distant recurrence in 1 patient (0.9%). The median follow-up in this study was only 28 months (13).
LYMPH NODE EVALUATION
In patients with DCIS, lymph node evaluation using sentinel lymph node biopsy (SLNB) is utilized in select cases. This includes patients who are undergoing mastectomy, since this precludes subsequent SLNB at a second operation, and physical exam or imaging findings that are concerning for invasive cancer, especially the presence of a mass lesion, DCIS that encompasses a large area on imaging (≥5 cm), and multicentric disease. We also perform SLNB for DCIS in cases where the location of the surgery may prevent a successful SLNB from being performed at a second surgery if invasive disease is identified.
BREAST CONSERVATION FOLLOWED BY RADIATION THERAPY FOR DCIS
The role of radiotherapy for patients with DCIS was established with the publication of four large prospective trials designed to address the effectiveness of BCS and RT for women with DCIS compared to BCS alone (5,14–16). The results of all four showed similar findings: the addition of RT resulted in an RR reduction of ipsilateral breast events (local failure) by 50%. With median follow-up intervals now of 13 to 17 years, omission of RT was associated with local failure rates of 23% to 35% compared to 10% to 20% in the irradiated arms. Of the local failures, approximately half were DCIS and half were invasive breast cancer. Despite higher local failure rates in surgery alone arms, survival is excellent and breast-cancer-related mortality is not different between arms. These findings are summarized in Table 4.1.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a meta-analysis of the randomized trials and found that radiotherapy reduced the absolute 10-year risk of any ipsilateral breast event (IBE) by 15.2%, an effect regardless of age at diagnosis, extent of BCS, use of tamoxifen, method of DCIS detection, margin status, focality, grade, the presence of comedonecrosis, architecture, or tumor size (17). They noted that the proportional reduction in ipsilateral breast events was greater in older than in younger women but did not significantly differ according to other available factors. However, after 10 years of follow-up, there was no significant effect on breast cancer mortality, mortality from causes other than breast cancer, or all-cause mortality, suggesting the effectiveness of salvage therapy or the long natural history of the disease.
FACTORS PROGNOSTIC FOR RECURRENCE
With the advent of breast screening and the trend for identification of smaller lesions, as well as the greater attention paid to surgical margin status (both factors associated with lower rates of IBE), there was general sentiment that the historic outcomes of BCS alone may not reflect current standards. Prognostic factors for recurrence were thus identified in an effort to characterize a population of women at sufficiently low risk such that radiotherapy could be safely omitted. These factors include: age, size, mode of detection, grade, architecture, focality, and margin status (5–7,18,19). In the Van Nuys Predictive Index, these factors were consolidated into a score, which predicted the risk of relapse after lumpectomy with or without RT. In unirradiated patients with scores of 4 to 6 there was a 10-year IBE rate of only 6% (20). These findings were confirmed with median follow-up of nearly 11 years showing a 5% event rate in “low-score” patients (21). The relative impact of these factors is depicted in Table 4.2.
Additionally, analysis of tumor genetics and molecular phenotype may further improve patient selection over classic pathologic and clinical factors. A 12-gene DCIS score (Genomic Health, Inc. Redwoods, California) was created from tissue samples of 327 patients treated with BCS alone in the ECOG 5194 trial. Patients with a low score (≤38) had a 10-year IBE rate of 12% compared to 25% and 27% in patients with intermediate (39–54) or high scores (≥55) (22). A study from Milan of over 1,100 women treated for DCIS with 10-year follow-up found Ki-67 labeling index and molecular phenotype to be significantly associated with recurrence risk. Five-year IBE rates were 9.4% for tumors with Ki-67 <14%, as compared to 10.3% for Ki-67 14% to 20% and 13% for Ki-67 >20% (23). The same study found 5-year IBE rates of 9.1%, 10.3%, and 15.3% for luminal A, luminal B/HER2-negative, and luminal B/HER2-positive subtypes, respectively. Notably, the use of clinical and pathologic factors seems to predict subgroups that have a low recurrence rate at a much lower cost.
BREAST CONSERVATION WITHOUT RADIOTHERAPY FOR SELECTED PATIENTS
Despite low local failure rates in individuals with favorable prognostic features, the EBCTCG meta-analysis still demonstrated an absolute reduction in the 10-year risk of IBE of 18.0% (12.1% vs. 30.1%) for women with negative margins and small low-grade tumors.
In a more recent SEER analysis of over 108,000 women, among patients who underwent BCS, radiotherapy was associated with a 2.4% absolute reduction in the risk of ipsilateral invasive recurrence at 10 years (2.5% vs. 4.9%) (24). Interestingly, the risk of dying of breast cancer increased after experiencing an ipsilateral invasive breast cancer (HR 18.1). However, receipt of radiotherapy after BCS was not associated with a change in breast-cancer-specific mortality at 10 years (0.8% vs. 0.9%).
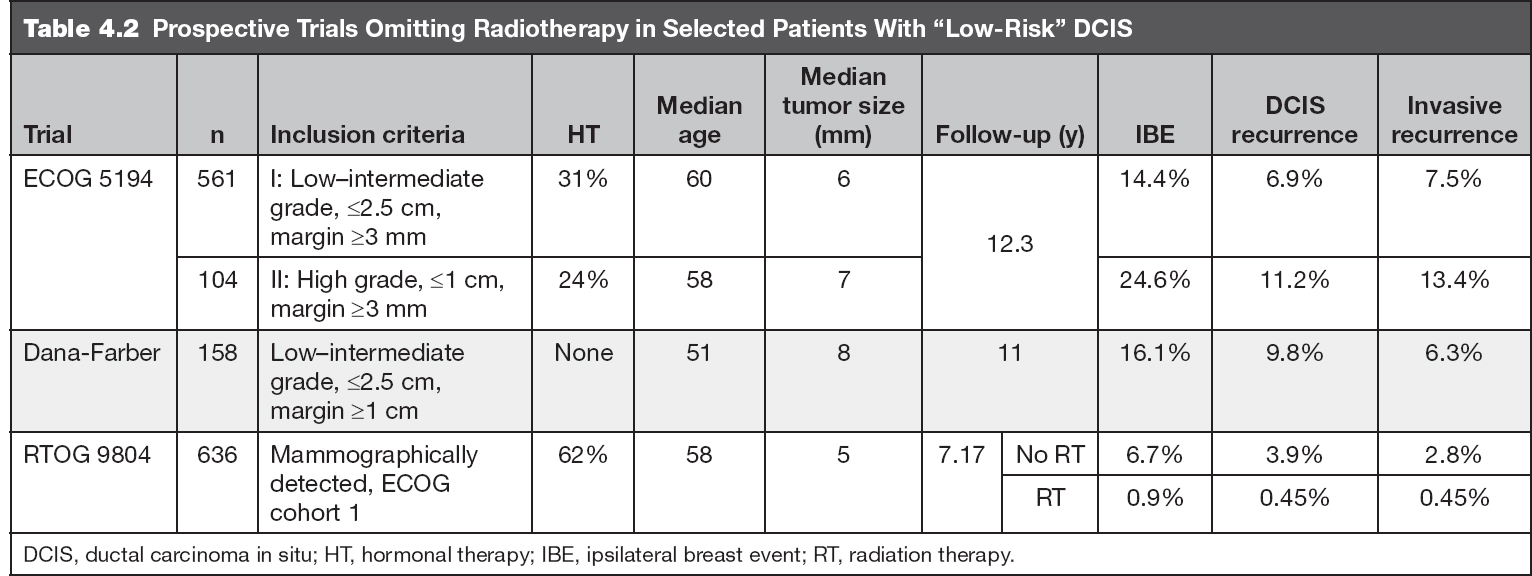
To answer the question of the clinical implications of BCS alone for patients with low-risk DCIS, several prospective trials were designed, which now have recently reported long-term follow-up (Table 4.1) (25–27). Paralleling the previous evidence, survival and breast-cancer-related mortality were excellent. In these well-selected women, BCS alone resulted in a 1% to 2% per year rate of IBEs. The local control rates of these trials are outlined in Table 4.3.
Within the ECOG-ACRIN 5194 trial, additional prognostic factors emerged: study cohort (HR 1.84 for cohort 2) and tumor size were both significantly associated with developing an IBE. Compared to tumors sized 5 mm or less, tumors 6 to 10 mm were associated with an HR of 1.42 and tumors greater than 10 mm with an HR of 2.11 for IBE. Variables not statistically significant were age, menopausal status, minimum negative margin width, method of detection, and tamoxifen use.
DECIDING WHEN IT IS SAFE TO WITHHOLD RADIOTHERAPY
In the previously discussed studies of patients with DCIS selected for favorable clinical and pathologic characteristics and treated with surgical excision without radiation, the risks of developing an IBE and an invasive IBE increased over time without plateau through 12 years of follow-up, confirming the known risk of late LRs. At the same time, RT substantially reduces local failures, but it does not impact the risk of metastases or breast cancer mortality.
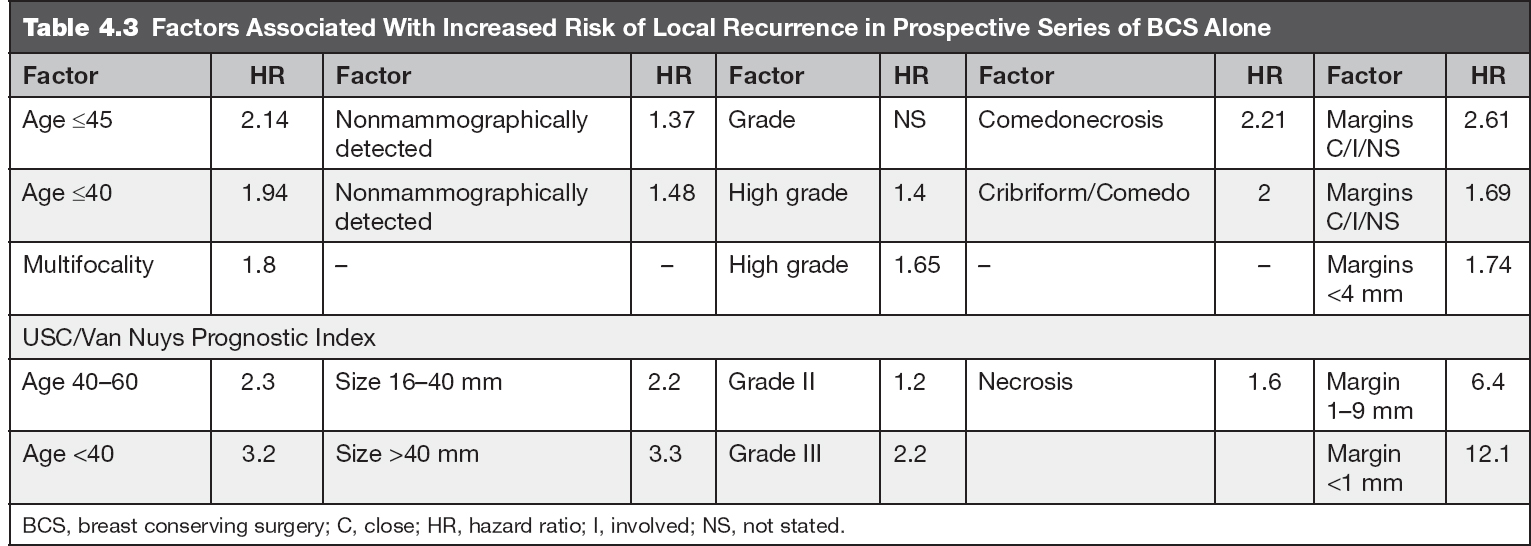
The decision to accept a 1% to 2% per year risk of LR must be individualized and weighed against comorbidities, comfort level, and life expectancy (Table 4.2). Potential clinical criteria necessary to quote this low event rate include the presence of all of the following: age older than 40 years; highest nuclear grade 1 or 2; maximum extent 2.5 cm or less; and margins greater than 2 mm or no tumor on reexcision (Table 4.3) (28).
RADIOTHERAPY OPTIONS
Adjuvant radiotherapy has consisted of tangential irradiation of the whole breast delivered over 5 weeks to a total dose of 50 Gy in once-daily fractions of 2 Gy. Several alternatives to standard whole breast irradiation (WBI) exist to reduce the duration of adjuvant RT, including hypofractionation and accelerated partial breast irradiation (APBI). A full discussion of all technical details is beyond the scope of this text; however, certain areas of interest are highlighted.
USE OF A BOOST
The use of a tumor bed boost (in addition to WBI) as a part of adjuvant radiotherapy for patients with invasive breast cancer has been found to improve local control; however, the role of a boost after BCS in patients with DCIS has not been addressed in prospective trials.
A meta-analysis of 12 studies including nearly 7,000 patients showed no difference in the risk of LR between the patients who received boost and no boost in the general cohort (29). A reduced risk for LR, however, was found for the use of a boost in patients with positive margins compared to no boost (OR 0.56). In a multi-institutional study included in the meta-analysis, 166 women received radiotherapy without a boost (median dose 50 Gy [range 40–60 Gy]) and 150 received radiotherapy with a boost (60 Gy [53–76 Gy]) (30). Local relapse-free survival at 10 years was 72% in those given radiotherapy without boost and 86% in those given radiotherapy with boost, despite more patients having positive or uncertain margins in the boost arm. Compared to radiotherapy without boost, radiotherapy with boost had an HR for local failure of 0.45 but no difference in overall survival. The use of a boost did not alter the proportion of invasive to in situ local recurrences (which was ~50:50, similar to the proportion in the randomized trials of BCS).
HYPOFRACTIONATION
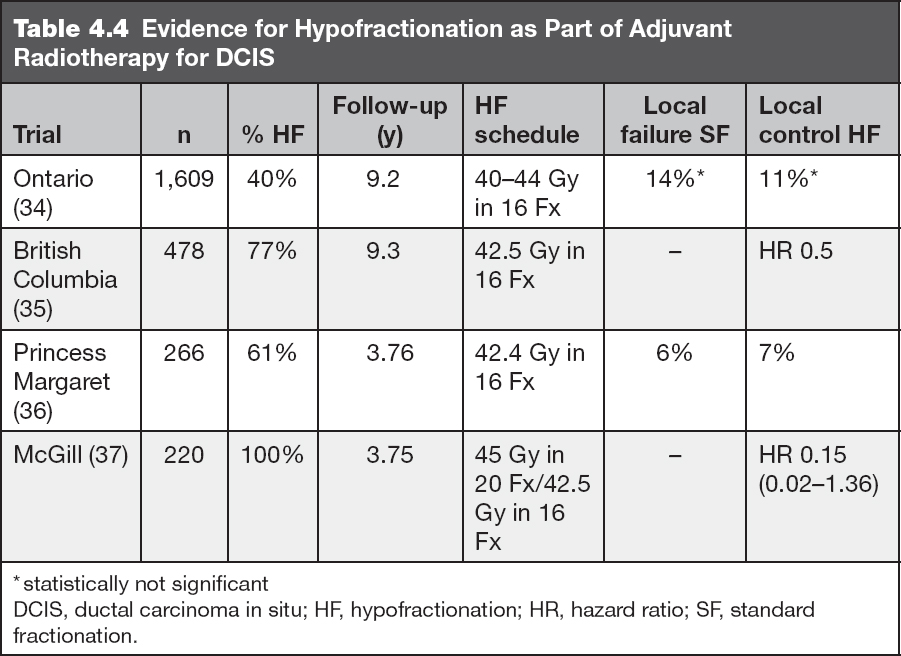
Hypofractionation is an alternative form of WBI where treatment duration is reduced, typically to 3 weeks. A commonly used schedule is 40 to 42.5 Gy delivered in 15 to 16 treatments, respectively. With over 10 years follow-up in the setting of invasive disease, no differences in outcomes or toxicity profiles have been noted when compared to standard fractionation in trials from the UK and Canada (31,32).
Hypofractionation for DCIS has also been the subject of meta-analysis (29). No difference was observed in LR rates between patients who received hypofractionated versus standard radiotherapy, paralleling the long-term data for treatment of invasive disease. Similar results have been noted in several studies, and there has been increased utilization of hypofractionation for DCIS in the United States (33). Reflecting this change, hypofractionation (42.5 Gy in 16 daily fractions) was allowed in the RTOG 9804 randomized study. The schedules and results for studies of hypofractionation in DCIS are depicted in Table 4.4.
ACCELERATED PARTIAL BREAST IRRADIATION
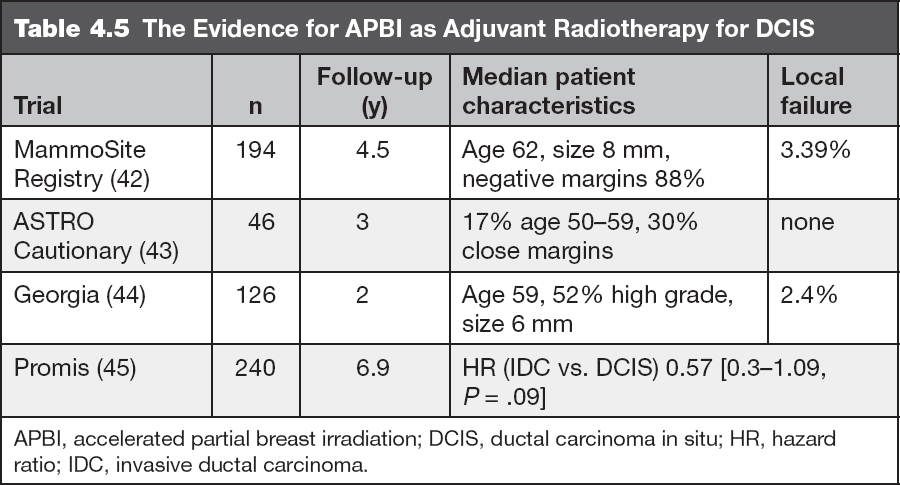
Another alternative to standard WBI is APBI, treating only the area surrounding the lumpectomy cavity, typically in 1 week or less. There are many ways to accomplish partial breast irradiation, including interstitial, intraoperative, intracavitary balloon-based, and external beam techniques. Randomized trials comparing APBI with WBI demonstrate equivalent clinical outcomes for selected patients with early-stage invasive disease (38).
Pure DCIS had been a “cautionary” criterion for the use of APBI as recommended by the American Society for Radiation Oncology (ASTRO) consensus statement (39); however, the American Brachytherapy Society APBI consensus statement now includes DCIS in the acceptable treatment category (40). In the setting of invasive disease, the presence of extensive intraductal component increases local failure rates (41). Data are emerging on patients with DCIS treated with APBI. With early follow-up, control rates appear acceptable. Results of several retrospective series are detailed in Table 4.5.
SYSTEMIC THERAPY FOR DCIS
DCIS is considered a localized disease with a very low risk of developing nodal or distant metastases and prognosis is excellent. Estrogen and/or progesterone receptor expression is high in DCIS (50%–70%) (46). Studies have shown that the significantly elevated risk of development of invasive carcinoma after diagnosis of DCIS persists for up to 25 years (47). After diagnosis of DCIS approximately 50% of breast cancer recurrences are invasive rather than noninvasive (48).
Chemotherapy has no role in management of patients with DCIS given the low likelihood of metastatic disease. Endocrine therapy is the mainstay of systemic treatment for DCIS following completion of surgery and as long as the patient has residual breast tissue.
SYSTEMIC ENDOCRINE THERAPY
Definition of ER expression in DCIS: The 2010 ASCO/CAP guidelines recommend classifying all cases with <1% positive cells by immunohistochemistry (IHC) as receptor negative. For DCIS with >1% positive cells, the percentage of positive cells is reported along with the intensity of staining.
ER-positive DCIS: That is completely excised, but where the patient has not had bilateral mastectomy and has residual breast tissue. We recommend chemoprevention with tamoxifen 20 mg daily for 5 years for premenopausal women and either tamoxifen 20 mg daily or anastrozole 1 mg daily for 5 years for postmenopausal women.
ER-negative DCIS: We do not routinely recommend chemoprevention with tamoxifen or an aromatase inhibitor such as anastrozole, although some women may still choose to take either of these agents to prevent subsequent ER-positive invasive or noninvasive breast cancers.
DCIS and BRCA1 or 2 mutation: The role and benefit of tamoxifen or aromatase inhibitors for chemoprevention for women with BRCA1 and 2 mutations and DCIS is largely unknown but it is likely similar to women without these mutations as long as other criteria for endocrine treatment are met as discussed previously. When these situations arise and the patient is not interested in prophylactic mastectomy surgery, we recommend chemoprevention with tamoxifen or anastrozole as long as other criteria for treatment are met (ER-positivity).
SYSTEMIC CHEMOPREVENTION TRIALS
RCTs have been conducted to investigate the benefit of tamoxifen or anastrozole after local treatments for DCIS.
Tamoxifen, a selective estrogen receptor modulator (SERM), is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effect is thought to be related to its ability to compete with estrogen for binding sites in target tissues such as the breast. Tamoxifen is Food and Drug Administration (FDA) approved at a dose of 20 mg daily for 5 years for prevention of invasive breast cancer recurrences in women with DCIS (FDA package insert).
Anastrozole is a triazole and type 2 nonsteroidal inhibitor of the aromatase enzyme; it binds reversibly to the enzyme substrate binding site and prevents the azole nitrogen’s interaction with the heme prosthetic group, allowing for exquisite potency for the binding site and specificity against the aromatase enzyme (49). Dr. Angela Brodie and collaborators established a tumor model in nude mice to simulate several aspects of the postmenopausal breast cancer patient (50). These studies showed that aromatase inhibitors are more effective than tamoxifen at reducing tumor volume and that the combination of an aromatase inhibitor plus tamoxifen did not improve the antiproliferative results obtained with the aromatase inhibitor alone. This was later substantiated clinically in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant clinical trial.
DCIS CHEMOPREVENTION TRIALS
National Surgical Adjuvant Breast and Bowel Project B-24
This was a double-blind, randomized trial of tamoxifen versus placebo in women with DCIS following treatment with lumpectomy and RT. The primary objective was to determine if 5 years of tamoxifen (20 mg/day) would reduce the incidence of invasive breast cancer in the ipsilateral or contralateral breast; 1,804 women were randomized to either tamoxifen 10 mg twice daily (N = 902) or placebo (N = 902) treatment for 5 years.
RESULTS
At 5-year follow-up, 83.3% (95% CIs [80.8, 85.8]) of patients who received placebo were event-free compared to 87.4% (85.1–89.6) of tamoxifen-treated patients and there was no difference in survival (51); with a median follow-up of 74 months, the incidence of invasive breast cancer was reduced by 43% among women assigned to tamoxifen versus placebo (44 cases vs. 74 cases); P = .004; RR = 0.57, 95% CIs [0.39, 0.84]). The risk of developing ipsilateral or contralateral DCIS was also reduced with 5 years of tamoxifen prevention.
ADVERSE EVENTS
The overall frequency of side effects was similar between the groups and 62.8% of placebo and 57.1% of tamoxifen patients reported no adverse events. Grade 4 toxic effects not usually associated with tamoxifen occurred with similar rates in the two groups. There was an increase in the rate of endometrial cancer in tamoxifen-treated patients (1.53 vs. 0.45 per 1,000 patients per year in the placebo group). No deaths from endometrial cancer occurred in the tamoxifen group. The rates of phlebitis/thromboembolism were low overall; deep venous thrombosis (DVT) 0.2% in placebo versus 1% in tamoxifen group and nonfatal pulmonary embolism, 1 case in placebo versus 2 in tamoxifen. No strokes were seen in the two treatment groups. Hot flashes occurred in both groups, placebo—N = 525 (59.0%) versus tamoxifen—N = 620 (69.6%) (51).
UK/ANZ DCIS Trial
This trial had a 2 × 2 factorial design, and 1,701 women were randomly assigned to radiation + tamoxifen, radiation alone, tamoxifen alone, or to no adjuvant treatment.
RESULTS
After a median follow-up of 12.7 years (IQR 10.9–14.7), 376 (163 invasive [122 ipsilateral vs. 39 contralateral], 197 DCIS [174 ipsilateral vs. 17 contralateral], and 16 of unknown invasiveness or laterality) breast cancers were diagnosed. Radiation reduced the incidence of all new breast events (HR 0.41; P < .0001), reducing the incidence of ipsilateral invasive disease (0.32; P < .0001) as well as ipsilateral DCIS (0.38; P < .0001). Tamoxifen reduced the incidence of all new breast events (HR 0.71; P = .002), reducing recurrent ipsilateral DCIS (0.70; P = .03) and contralateral tumors (0.44; P = .005), but had no effect on ipsilateral invasive disease (0.95; P = .8). Data on adverse events except cause of death was not collected for this trial (16).
A systematic review and meta-analysis of postoperative tamoxifen following surgical resection of DCIS using a fixed effect model was done (52).
Data on local DCIS recurrence, new invasive breast cancer, distant disease, mortality, and adverse effects were extracted from RCTs comparing tamoxifen after surgery for DCIS (regardless of ER status), with or without adjuvant radiotherapy; 2 RCTs (16,51) were included. Tamoxifen after surgery for DCIS reduced recurrence of ipsilateral DCIS (HR 0.75; 95% CIs [0.61, 0.92]) and contralateral DCIS (RR 0.50; 95% CIs [0.28, 0.87]). Contralateral invasive breast cancer was reduced (RR 0.57; 95% CIs [0.39, 0.83]), and there was a trend toward decreased ipsilateral invasive breast cancer (HR 0.79; 95% CIs [0.62, 1.01]). The number needed to treat in order for tamoxifen to have a protective effect against all breast events is 15. There was no evidence of a difference in all-cause mortality (RR 1.11; 95% CIs [0.89, 1.39]).
Aromatase Inhibitors Versus Tamoxifen
IBIS-II DCIS– was a double-blind, multicenter, randomized placebo-controlled trial. Women with locally excised, hormone-receptor-positive DCIS were eligible and randomly assigned in a 1:1 ratio to receive anastrozole 1 mg daily or tamoxifen 20 mg daily for 5 years. The primary end point was all recurrence, including recurrent DCIS and new contralateral tumors; 2,980 postmenopausal women from 14 countries were randomly assigned to receive anastrozole (1,449 analyzed) or tamoxifen (1,489 analyzed) (53).
RESULTS
With a median follow-up of 7.2 years (IQR 5.6–8.9), 144 breast cancer recurrences were seen, there was a statistically significant difference in overall recurrence (67 recurrences for anastrozole vs. 77 for tamoxifen; HR 0.89; 95% CIs [0.64, 1.23]). Anastrozole treatment was noninferior but not superior to tamoxifen (upper 95% CI <1.25). There was no difference in deaths between the two treatment groups (53).
ADVERSE EVENTS
The number of any adverse events was similar between anastrozole (1,323 women, 91%) and tamoxifen (1,379 women, 93%). As expected, the side-effect profiles of the two drugs were different. More fractures, musculoskeletal events, hypercholesterolemia, and strokes were observed with anastrozole, while more muscle spasm, gynecological cancers and symptoms, vasomotor symptoms, and deep vein thromboses were observed with tamoxifen (53).
National Surgical Adjuvant Breast and Bowel Project B-35
Postmenopausal women with hormone positive DCIS treated by lumpectomy with clear resection margins and WBI were enrolled and randomly assigned (1:1) to receive tamoxifen 20 mg per day (with matching placebo) or anastrozole 1 mg per day (with matching placebo) for 5 years. Randomization was stratified by age (<60 vs. ≥60 years). The primary outcome was breast-cancer-free interval, defined as time from randomization to any breast cancer event (local, regional, or distant recurrence, or contralateral breast cancer, invasive disease, or DCIS), analyzed by intention to treat (54).
RESULTS
In total, 3,104 women were randomized to the two treatment groups (1,552—tamoxifen and 1,552—anastrozole); with median follow-up of 9 years (IQR 8.2–10.0); 212 breast-cancer-free interval events occurred: 122 in the tamoxifen group and 90 in the anastrozole group (HR 0.73, 95% CIs [0.56, 0.96]; P = .0234). There was also a significant interaction between treatment and age group (P = .0379), showing that anastrozole was superior only in postmenopausal women younger than 60 years of age. In this trial, compared to tamoxifen, anastrozole treatment provided a significant improvement in breast-cancer-free interval, mainly in women younger than 60 years of age (54).
ADVERSE EVENTS
Adverse events were similar between anastrozole and tamoxifen, except for thrombosis or embolism; 17 grade 4/5 events in the tamoxifen versus 4 in the anastrozole group were noted.



