Mechanical Devices and Embolectomy
HervÉ Decousus
Patrick Mismetti
Nicolas Meneveau
VENA CAVA FILTERS AND VENOUS THROMBOEMBOLISM
Interruption of the vena cava by filter insertion was initially suggested by Armand Trousseau in 1868, and these devices have been used since the 1970s to reduce the risk of pulmonary embolism (PE).1, 2 These devices have been used for the treatment of venous thromboembolism (VTE) either alone, in patients with a contraindication to anticoagulation, or in combination with conventional anticoagulation, especially in case of failure of the antithrombotic therapy. Inferior vena cava (IVC) filters could also be proposed as VTE prophylaxis in certain patient populations at high thromboembolic risk. Despite these rather limited indications and despite current guidelines, IVC filters are frequently used especially in North America with 49,000 insertions in the United States in 1999.3, 4 Forty-five percent of the filters are inserted for patients with deep vein thrombosis (DVT), 36% for those with PE, and 19% are used as primary prophylaxis.3 An increasing proportion of all filter insertions are outside the recommendations of current guidelines.5 The use of these devices has been extensively studied with more than 2,000 publications during the past 10 years. However, these publications are mainly case reports or retrospective cohorts. Several prospective cohort studies with a limited number of patients have been performed, and only two randomized clinical trials have been published6, 7: the first comparing IVC filter plus anticoagulant therapy with anticoagulant therapy alone in patients with proximal DVT and no contraindication to anticoagulation6 and the second comparing two types of IVC filters in patients requiring filters for different reasons.7
Different Types of Filters
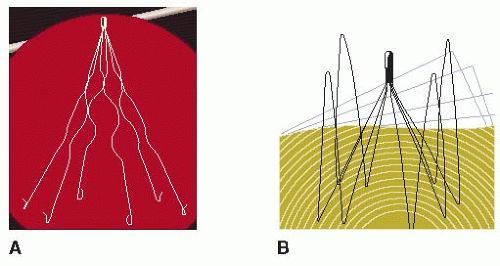
Several types of IVC filters have been developed, and at least 10 variants of vena cava filters are currently in use in North America and Europe. The original filters were permanent, with no possibility of removal, such as the Greenfield (Boston Scientific, Boston, MA, USA), Bird’s Nest (Cook, Bloomington, IN, USA), Venatech (B Braun, Boulogne, France), and Simon nitinol (Bard, Tempe, AZ, USA) filters (FIGURE 114.1).
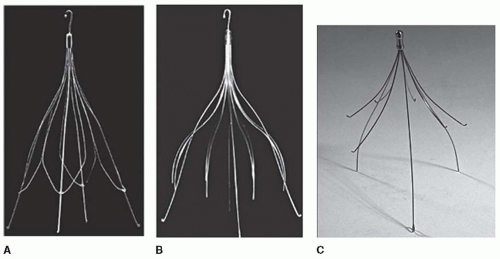
One of the main complications of these filters is a twofold increase in DVT recurrences.6, 8 Due to this risk, the filter placement requires prolonged anticoagulation, and despite this, the rate of filter thrombosis, of new lower extremity DVT, and of new PE was 12%, 20%, and 5%, respectively.9 This complication may occur late (≥3 months after filter insertion)6 and could be avoided if the filter is retrieved, since the indications for IVC filters are mainly temporary. Accordingly, temporary filters were then developed, but they need a permanent venous access to be retrieved (Tempofilter II, B. Braun, Boulogne, France). This permanent venous access could be associated with septic complications, and this reduced the duration of IVC filter implantations. More recently, retrievable filters, such as the Gunther Tulip (Cook, Bloomington, IN, USA), Recovery (Bard, Tempe, AZ, USA), OptEase (Johnson and Johnson, Cordis Endovascular, Miami, FL, USA), and ALN (ALN Implants Chirurgicaux, France) filters, have been developed (FIGURE 114.2). These filters can be left in place indefinitely or retrieval can be performed several weeks and up to 1 year after insertion.10
Complications of IVC Filters
Before discussing their efficiency and potential indications, it seems important to note that apart from the thromboembolic complications mentioned above, the iatrogenic effects of these filters can be serious, and the incidence of these complications is not clearly established. Indeed the occurrence of adverse events after filter implantation is not negligible, as shown in several cohorts.11 Moreover failures of filter removal can reach 12% in observational studies.11
These complications are listed in Table 114.1.
Recommendations for Use
As with all invasive procedures, insertion of a vena cava filter may be associated with a risk of serious adverse events,3 justifying careful consideration of the benefit-to-risk ratio for all indications. Due to the limited data currently available regarding the benefit-to-risk ratio of these devices, their use even for validated indications should be restrictive.
The eighth edition of the American College of Chest Physicians (ACCP) guidelines published in 2008 recommends12
For the use of IVC filters in patients with acute proximal lower extremity DVT, in whom anticoagulant therapy is not possible because of the risk of bleeding (grade 1C)
Against the routine use of filter in addition to anticoagulants for most patients with PE and for patients with proximal DVT (grade 1A)
Against the routine use of filter as primary prophylaxis in trauma patients (grade 1C) and in spinal cord injury (grade 1C)
Despite these recommendations, filters are increasingly prescribed especially in the United States either to prevent or to treat VTE.13 This is probably due to potential indications that are insufficiently assessed. These potential indications correspond in particular to patients supposed to be at risk of VTE despite anticoagulant therapy and/or to be at high risk of bleeding while receiving anticoagulants.4, 13
Recommended and Potential Indications of IVC Filters
Treatment of VTE
For this indication, there are considerable discrepancies between the United States and Europe in terms of frequency of use. Two North American cohorts showed that a filter4, 13, 14 was inserted in about 15% of patients hospitalized for an episode of VTE compared with only 2% of patients in a similar cohort in Europe.15
In one of the North American cohorts,4 three experts in the subject reviewed each of the indications for the filters, and only 50% of the indications were considered to comply with ACCP guidelines. The indications outside of the guidelines were essentially related to the perception of a high risk of bleeding or to a recurrence of VTE despite anticoagulant therapy.
Treatment of VTE in Patients with a High Risk of Bleeding
In patients presenting with a recent VTE episode and a contraindication for anticoagulant treatment, the risk of PE recurrence or PE-related death may reach 20%.16, 17 With this risk in mind, it is not ethically acceptable to perform a randomized trial to validate use of the filter.
There is, however, a European cohort study that supports this indication for the IVC filter. In a population of 17,368 VTE patients, the incidence of major hemorrhagic incidents was 2.3%, and the mortality rate for the patients who experienced bleeding was 33%. Among these patients with a hemorrhagic incident, a filter was inserted in 9% and multivariate analysis identified the filter as the only independent protective factor with a reduced risk of death after a hemorrhagic incident, with an odds ratio of 0.2 (95% confidence interval [CI], 0.1 to 0.6).18
These results would at first sight justify use of the vena cava filter in all VTE patients with a hemorrhagic incident contraindicating anticoagulant therapy. Indeed, when these hemorrhagic incidents are transient or curable, use of a retrievable filter is fully justified, provided that removal of the filter is effectively scheduled.
Treatment of VTE in Patients at a High Risk of Fatal PE
IVC filters could be used when the risk of death due to PE is high (>5% at 3 months) despite anticoagulation,4 that is,
In hemodynamically unstable patients with PE
In patients at risk of recurrence of life-threatening PE, such as those with chronic pulmonary heart disease, especially if a surgical treatment if proposed
In these specific situations, indications for IVC filter placement are intuitive, and a randomized clinical trial is unrealistic and not feasible. In the absence of more convincing data, the indications are not discussed in the most recent guidelines.12
Treatment of VTE Recurrences Despite Anticoagulant Treatment
In VTE patients presenting with a recurrent PE in spite of optimal anticoagulant treatment, use of vena cava filters appears to be appropriate. However, there are no scientific data to support this indication. Moreover, it is necessary in this case to discuss the notion of optimal anticoagulant treatment.
For example, in studies in cancer patients with an episode of VTE, continued administration of low molecular weight
heparin for 3 to 6 months seemed to be more effective than vitamin K antagonists (VKAs), even when the international normalized ratio (INR) was optimally controlled.19, 20 Thus, for recurrent PE during VKA therapy with an INR between 2 and 3, it may be more appropriate to resume heparin treatment rather than to use a vena cava filter. It is likely that the arrival of the new anticoagulants will provide another alternative to VKAs and filters in this situation.
heparin for 3 to 6 months seemed to be more effective than vitamin K antagonists (VKAs), even when the international normalized ratio (INR) was optimally controlled.19, 20 Thus, for recurrent PE during VKA therapy with an INR between 2 and 3, it may be more appropriate to resume heparin treatment rather than to use a vena cava filter. It is likely that the arrival of the new anticoagulants will provide another alternative to VKAs and filters in this situation.
Table 114.1 Different types and rates of adverse events associated with filter implantation | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Treatment of VTE in Patients at High Risk of Recurrences Despite Anticoagulant Treatment
The only well-conducted randomized trial, the PREPIC study was designed for patients with this indication.6 In this study, patients with a proximal DVT were randomly allocated to receive either anticoagulation with insertion of a filter or anticoagulant treatment alone. The filters assessed in this study were permanent versions. The results after 8 years of follow-up showed a significant reduction in the risk of PE (hazard ratio [HR] 0.37; 95% CI, 0.17 to 0.79; P = 0.008), but this beneficial effect was accompanied by a significant rise in the risk of DVT in the lower limbs (HR 1.57<; 95% CI, 1.02 to 2.27; P = 0.042) and notably with a 13% risk of filter thrombosis.21 These contradictory results justify the fact that the guidelines do not recommend routine use of filters.12
The beneficial effects with respect to PE are evident essentially during the 1st 3 months of treatment, while the negative effects are observed mostly after the sixth month.6 Therefore, a retrievable filter left in place for only 3 months could achieve the beneficial effects regarding PE while avoiding the extra risk of DVT recurrence. In addition, in order to optimize the beneficial effects of the filter, it could be inserted in patients with a higher risk of PE than in the PREPIC population. In fact, when recruited to the study, 38% of the PREPIC patients presented with DVT alone without any initial PE,6 whereas the presence of an initial PE multiplies by five the risk of a fatal PE at 3 months compared to isolated DVT.8, 22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree