MARKERS OF BONE METABOLISM
Part of “CHAPTER 56 – MARKERS OF BONE METABOLISM“
Assays of biochemical markers of bone turnover are noninvasive and, when the results are applied and interpreted correctly,
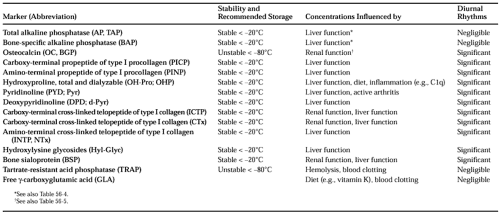
are very helpful tools in the assessment of metabolic bone disease. The various serum and urinary components used as markers of bone turnover include enzymes released by bone cells and peptides derived from the skeletal matrix during bone formation or bone resorption. For clinical purposes, bone biomarkers are usually classified according to the metabolic process they are considered to reflect; that is, bone formation or bone resorption (see Table 56-1 and Table 56-2). Some components, such as the serum amino-terminal procollagen type I propeptide and urinary hydroxyproline, are derived from anabolic and catabolic processes and are, therefore, influenced by the rate of bone formation and bone resorption. Other markers, such as the bone isoenzyme of alkaline phosphatase or the pyridinium cross-links of collagen, are more specific to individual metabolic processes.
are very helpful tools in the assessment of metabolic bone disease. The various serum and urinary components used as markers of bone turnover include enzymes released by bone cells and peptides derived from the skeletal matrix during bone formation or bone resorption. For clinical purposes, bone biomarkers are usually classified according to the metabolic process they are considered to reflect; that is, bone formation or bone resorption (see Table 56-1 and Table 56-2). Some components, such as the serum amino-terminal procollagen type I propeptide and urinary hydroxyproline, are derived from anabolic and catabolic processes and are, therefore, influenced by the rate of bone formation and bone resorption. Other markers, such as the bone isoenzyme of alkaline phosphatase or the pyridinium cross-links of collagen, are more specific to individual metabolic processes.
Most of the compounds that are used as markers of skeletal metabolism are not unique to bone, as they also occur in other tissues (see Table 56-1 and Table 56-2). Few or perhaps none of the available markers is absolutely specific for bone. Moreover, most serum and urinary indices are influenced by nonskeletal diseases, such as inflammatory conditions, malignancies, and chronic renal or hepatic failure. Changes in biochemical markers of bone metabolism are, therefore, not disease specific; abnormal results should always be interpreted within the context of the clinical picture. With these notions in mind, one may use bone markers for the following purposes:
To evaluate bone turnover in individual patients and disorders
To predict future bone loss and hip fractures in larger cohorts
To select therapy for individual patients
To predict therapeutic response in individual patients
To monitor therapeutic response and efficacy in individual patients
MARKERS OF BONE FORMATION
All bone formation markers are products or enzymes released by active osteoblasts and are generally measured in serum or plasma. The most commonly used markers of bone formation are alkaline phosphatase, OC, and the propeptides of type I collagen.
ALKALINE PHOSPHATASE
The alkaline phosphatases form a family of isoenzymes that are found in a multitude of tissues, including bone (e.g., in osteo-blasts), liver, intestines, kidney, and placenta.1 The various iso-forms can be differentiated by their carbohydrate content. Several methods have been established to determine specifically the enzyme fraction derived from osteoblasts, including selective denaturation, inhibition or activation, electrophoretic separation, and immunologic quantification.2
In healthy adults, ˜50% of the total serum alkaline phosphatase (TAP) activity is derived from osteoblasts, whereas the other half is usually of biliary-hepatic origin. Particularly in elderly patients, elevated serum TAP activities are more often the result of hepatic afflictions than of skeletal diseases (Table 56-4). Therefore, serum TAP levels should be used as an index of bone formation only if an impairment of liver and biliary function can be excluded. In contrast, the bone-specific isoenzyme of alkaline phosphatase (BAP) is located exclusively in the osteoblast membrane, from which it is released into the circulation on osteoblast activation. Compared to the total enzyme pool, serum BAP levels are clearly less affected by nonskeletal disorders and therefore more specific for changes in bone formation. In the clinical setting, however, the diagnostic sensitivity of serum BAP is not superior to that of serum TAP.2
Total alkaline phosphatase is the classic laboratory marker of skeletal activity in Paget disease of bone. Serum levels of the total and bone-specific enzymes are markedly increased when the disease is active, but are normal or only slightly elevated when patients have monostotic, mild polyostotic, or inactive disease. In certain cases, determination of serum BAP instead of TAP may help identify patients with very mild disease. Rather high levels of serum TAP or BAP are typically seen in patients with involvement of the skull, and sometimes in cases with sarcomatous degeneration of pagetic bone. Serial measurements of serum TAP are helpful to monitor a therapeutic response and to detect a recrudescence of disease activity.
Primary hyperparathyroidism was previously often associated with significantly elevated levels of serum alkaline phos-phatase, indicating gross bone involvement. However, as the clinical profile of the disorder has evolved toward a predominance of cases with asymptomatic hypercalcemia, marked elevations in serum alkaline phosphatase are rarely seen (see Chap. 58).3 The expectation that serum BAP may be a more sensitive parameter of bone involvement in asymptomatic primary hyperparathyroidism has not yet been met.2
For the use of serum TAP and/or BAP measurements in osteoporosis, see last section of this chapter.
In osteomalacia, total and bone-specific enzyme activities are markedly elevated; the determination of serum alkaline phosphatase is often a clue to the diagnosis of this disorder. In renal osteodystrophy, elevated levels of serum alkaline phos-phatase may be indicative of progressive skeletal involvement and, particularly in patients on chronic hemodialysis, should prompt appropriate diagnostic and therapeutic measures.
Serum levels of TAP and BAP may be elevated in primary and secondary skeletal malignancies. High serum activities are often found in osteoblastic bone metastases and after multiple fractures. In contrast, the alkaline phosphatase level is usually low in multiple myeloma. Because of its rather low sensitivity and specificity, serum alkaline phosphatase is not recommended as a tumor marker for clinical use.
OSTEOCALCIN
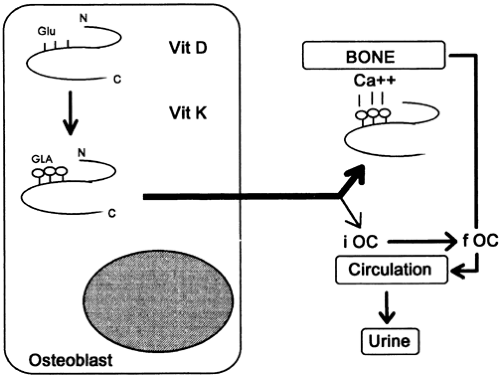
Osteocalcin (OC, or bone Gla protein), a small, GLA–rich peptide synthesized by osteoblasts, is one of the major noncollagenous proteins of the bone matrix.4,5 The molecule is found exclusively in mineralized tissues, and although its precise function is unknown, one obviously important property of OC is its high affinity for hydroxyapatite. Interestingly, OC-deficient knock-out mice have increased cortical and trabecular bone thickness, and mechanically more stable bones than wild-type mice.6 Therefore, during mineralization, OC may act via a negative feedback mechanism.
After its secretion by activated osteoblasts, the major fraction of OC is incorporated into the extracellular matrix. However, 15% to 30% of the newly synthesized peptide is released into the general circulation, where it may be detected and quantified by immunoassay (Fig. 56-2). Although serum levels of circulating OC correlate well with the rate of bone formation,7 significant drawbacks are encountered in the practical use of this marker. Serum OC levels are strongly affected by the pronounced thermal instability of the molecule, the specificity of the antibodies, and factors such as hormonal status, renal function, age, and sex4,4a (Table 56-5 and Table 56-6).
With the exception of Paget disease of bone, in which serum OC levels are often normal, most conditions with increased bone and mineral formation are characterized by elevated serum concentrations of OC. This is the case in primary hyperparathyroidism and hyperthyroidism, in which serum OC levels correlate with the extent of bone involvement.8 In healthy women, a transient, two-fold increase of serum OC is seen during early menopause, and individual values appear to correlate with the rate of bone loss in this population.9,10 In contrast, a wide range of values is observed in overt postmenopausal osteoporosis, which may be caused in part by the heterogeneity of the disease.
A different situation may be present in the elderly and in patients with senile osteoporosis. In this population, the effect of aging and possibly a deficiency of vitamins K and D may lead to an impairment in the γ-carboxylation of OC and ultimately to an increase in the proportion of partially undercar-boxylated circulating OC. Several studies now indicate that the proportion of undercarboxylated OC in serum may be an important determinant of femoral bone mineral density in elderly women, because high serum levels of undercarboxy-lated OC are associated with a low bone density at the hip and an increased risk of hip fractures.11 (See also the last section of this chapter.)
Serum OC levels are often elevated in untreated osteomalacia, in which they correlate with histomorphometric indices of osteoid formation.12 However, serum OC levels are usually much less elevated than the corresponding serum TAP or BAP levels.
In advanced renal failure (i.e., glomerular filtration rate of <20–30 mL/minute/1.73 m2), the accumulation of intact OC
and its various immunoreactive fragments may lead to very high serum levels, even in the absence of significant skeletal disease (see Table 56-6). OC levels are not affected by hemodialysis. Because of impaired renal clearance and increased bone turnover, serum OC usually is elevated in patients with renal osteodystrophy.
and its various immunoreactive fragments may lead to very high serum levels, even in the absence of significant skeletal disease (see Table 56-6). OC levels are not affected by hemodialysis. Because of impaired renal clearance and increased bone turnover, serum OC usually is elevated in patients with renal osteodystrophy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree