Low-risk prostate cancer
All of T1 or T2a, Gleason < 7, PSA < 10
Intermediate-risk prostate cancer
Between low- and high-risk groups
High-risk prostate cancer
Any of T3 or T4, Gleason > 7, PSA > 20
In general the treatment options for localised disease have comparable outcomes, and therefore management recommendations require assessment of comorbidities, performance status and patient choice. The management options are discussed in the following sections.
3.2 Active Surveillance
This conservative modality avoids overtreatment for low-risk disease, which is increasingly pertinent in the era of routine PSA testing [3]. It seeks to reduce the burden of side effects without compromising overall survival. Patients are closely observed using a multimodality approach (biochemical, radiological, histopathological). Approximately 30% of patients on active surveillance (AS) will subsequently require radical curative treatment, and 10-year prostate cancer-specific survival rates approach 100% [4, 5].
Indication:
Low-risk prostate cancer and selected men with intermediate risk disease
Suitable for radical treatment if indicated [1]
Side effects:
Psychological
3.3 External Beam Radiotherapy
External beam radiotherapy (EBRT) has an established role in the radical treatment of localised prostate cancer [6]. Developments have focused on maximising the tumour dose whilst limiting irradiation of normal tissues. 3D conformal therapy has evolved into image-guided (IG) intensity modulated radiotherapy (IMRT) as the standard of care [7] (see Fig. 3.1).
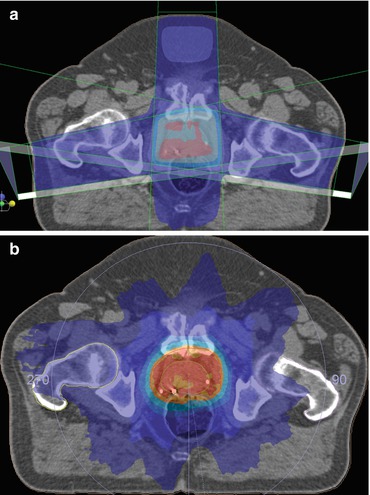

Fig. 3.1
(a) IMRT tightly conforms the high-dose radiotherapy volume to the target by modifying both the shape and fluence of the radiation field in real-time during treatment delivery. (b) The location of the prostate varies relative to the surrounding pelvic anatomy due to changes in the degree of rectal distension and bladder filling. This can impact on tumour control [8]
Hypofractionated techniques are under investigation and may offer a greater therapeutic ratio than standard fractionation [7, 9–14].
Androgen deprivation therapy (ADT) can be given in combination with radiotherapy. It achieves cytoreduction, allowing the use of smaller treatment fields, and potentiates tumour cell kill [9, 10]. Adjuvant ADT also improves overall survival in patients with high-risk disease [11–13]. The optimum duration of ADT relative to the risk of the disease is yet to be established [1].
EBRT can also be given as adjuvant or salvage therapy following radical prostatectomy (RP). Patients likely to have residual disease in the prostate bed (pT3; positive surgical margins; persistently detectable PSA; slowly rising PSA) are suitable candidates [14, 15].
Indications:
All prostate cancer risk categories
Post-operatively for high-risk disease
Side effects:
Acute: cystitis, diarrhoea, proctitis, rectal bleeding
Late: change in bowel habit, proctitis, impotence, secondary malignancy (rare)
3.3.1 Brachytherapy
Trans-perineal low-dose rate (LDR) brachytherapy involves the insertion of Iodine-125 (or Palladium-103) seeds into the prostate under ultrasound guidance. For low-risk disease the efficacy is at least comparable to EBRT/RP [16]. For intermediate- and high-risk patients, combination therapies involving EBRT with a brachytherapy boost (with/without ADT) may offer superior treatment outcomes [17].
Indications:
Selected patients with low and intermediate risk disease
High-dose rate (HDR) brachytherapy is delivered via a temporary iridium-192 implant inserted through hollow catheters placed in the prostate. In combination with EBRT, it can improve biochemical relapse-free survival and prostate cancer-specific survival compared to EBRT alone (intermediate-/high-risk disease) through dose escalation. Toxicity profiles are similar to EBRT alone [20, 21].
3.3.2 Radical Prostatectomy
Radical prostatectomy reduces prostate cancer-specific and all-cause mortality when compared to watchful waiting [22]. However recent evidence has not demonstrated benefits for all patients [23]. The incidences of post-operative complications, positive surgical margins and late urinary complications are reduced when performed by “high volume” surgeons in “high volume” centres [24, 25].
Surgery can now be performed open, laparoscopically or with robotic assistance (RALP). Nerve-sparing techniques have reduced the incidence of impotence but are only considered where they are not predicted to compromise surgical margins [26]. Extended lymph node dissection is considered for high-risk cases [7, 27].
Indications [7]:
Low and intermediate risk disease
Life expectancy >10 years
Selected patients with high-risk disease
3.4 Metastatic Disease
The first-line treatment for patients with metastatic disease is ADT either with orchidectomy, luteinising hormone-releasing hormone (LHRH) agonists or gonadotrophin-releasing hormone (GnRH) antagonists [17]. There is established evidence for early addition of docetaxel for suitable patients, although there is debate over whether this should just be for those with a high burden of disease [28, 29].
Management of “castration-resistant” metastatic prostate cancer (mCRPC) depends on disease burden, disease location, symptoms, PSA velocity, patient fitness, response to previous treatments and patient preference. There remains debate regarding optimum treatment sequencing and the individual contribution of each agent to overall survival, but the following agents have all demonstrated efficacy.
3.4.1 Corticosteroids
3.4.2 Cytotoxic Chemotherapy
Docetaxel + prednisone is the first-line chemotherapy agent for patients with a good performance status and is beneficial in terms of overall survival, quality of life and pain control [32]. Cabazitaxel is a second-line cytotoxic agent, also associated with a survival benefit and improved pain control, particularly in patients whose cancer progresses on/shortly after completing docetaxel therapy [33].
3.4.3 Androgen Receptor Pathway Targeted Agents
In castrate-resistant disease the androgen receptor (AR) pathway remains a useful target. Abiraterone acetate (a CYP-17 inhibitor) inhibits androgen biosynthesis in the adrenal glands, the tumour and the testes. It is administered with prednisone to minimise mineralocorticoid side effects. Enzalutamide targets multiple steps in the AR signalling pathway and, unlike other anti-androgens, has no partial-agonist action. Both agents have demonstrated efficacy in both the pre- and post-docetaxel settings, improving biochemical and radiological control, delaying deterioration in quality of life and improving survival [34–37].
3.4.4 Other Agents
Other treatment options include diethystilboestrol [38] and more recently Alpharadin (radium-223), an alpha emitter which targets bone metastases with alpha particles. The latter is associated with survival, quality of life and pain control benefits [39].
In prostate cancer, bisphosphonates are used to reduce/delay skeletal-related events (e.g. zoledronic acid) [40]. Denosumab is a monoclonal antibody that targets RANK ligand-mediated activation of osteoclasts. It is superior to zoledronic acid in delaying or preventing SREs [41].
Key Points
Three predictive factors are used to risk stratify localised prostate cancer: Gleason grade, PSA and T-stage. They predict the risk of lymph node involvement, treatment failure and death from prostate cancer.
Treatment options for localised disease have comparable outcomes; therefore management recommendations require assessment of comorbidities, performance status and patient choice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree