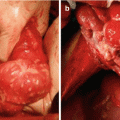
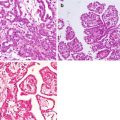
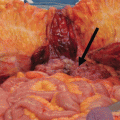
Fig. 7.1
WHO classification of tumors of the uterine corpus and histopathological subtypes of uterine sarcomas (4th edition, 2014) [25] (*Carcinosarcoma is no longer considered a uterine sarcoma. WHO World Health Organization)
Carcinosarcoma (CS) remains classified as mixed epithelial and mesenchymal tumors in the updated WHO classification 4th edition; however, it is still regarded as a subset of endometrial carcinoma based on its pattern of spread and the fact that mutation profiles resemble endometrial serous and endometrioid carcinomas [26–28]. Sarcomatous transdifferentiation of the underlying endometrial carcinoma has been hypothesized [29–31], and CS is now considered and treated as a high-grade epithelial tumor [32]. Therefore, CS should not be categorized as uterine sarcomas.
LMS is a malignant smooth-muscle tumor and is the most common type of US with an incidence of 63% [27, 33]. Epithelioid and myxoid LMS are two histopathological variants that differ from the ordinary spindle cell leiomyosarcoma [25]. LMS has a poor prognosis even in tumors confined to the uterus with only a slightly better 5-year survival in myxoid LMS (73%), compared to ordinary LMS (49%) [27, 34]. LMS at stage II has a 5-year survival of 25%, with no patients alive at 5 years when tumor spreads outside the pelvis [34]. Recurrence rates are from 53% to 71% [2, 35].
Endometrial stromal sarcomas (ESS) are the second most common mesenchymal tumor of the uterus (21%) and resemble the endometrial stroma in the proliferative phase [25, 33]. Currently, molecular studies have identified genetic signatures that support a subdivision of ESS into low- and high-grade entities, despite no typical histopathological features in this classification [36]. New evidence support that JAZF1-SUZ12 (formerly JAZF1-JJAZ1) gene fusion caused by t(7;17)(p15;q21) translocation is present in low-grade ESS (LG-ESS) [15, 37–39], while YWHAE-FAM22A/B translocation defines high-grade ESS (HG-ESS) [36]. Patients with LG-ESS and FIGO stages I and II have a 5-year survival rate greater than 90%, while patients with advanced stages of the disease have significantly lower rates between 40% and 50% [15, 40]. Recurrence in LG-ESS is common and greater in advanced stage disease. Patients with HG-ESS usually present with advanced stage disease, and progression is more common compared with LG-ESS. Mean OS is between 1 and 2 years [1]. At present, molecular analyses are not used in routine pathologic evaluation, but are helpful to classify difficult cases and are potential future therapeutic targets.
Undifferentiated uterine sarcoma (UUS) arises in the endometrium or myometrium with high-grade cytological features and no specific type of differentiation [25]. UUS lacks resemblance to the proliferative phase of endometrial stroma and exhibits a complex karyotype with no specific translocation [36]. It is a rare tumor, and the diagnosis is one of exclusions of the more commonly encountered differentiated US, such as LMS and AS [36]. When patients are diagnosed with this aggressive tumor, it is usually in the advanced stage (>60%) and has worse survival when compared with LG/HG-ESS. Five-year survival is <50%, and even patients with stage I tumors die within 2 years [25].
Adenosarcoma (AS) is a mixed tumor in which the epithelial component is benign or atypical and the stromal component is low-grade malignant [25].The stromal component usually are low grade (approximately 90% of cases), but when at least 25% of the tumor corresponds with high grade, it is classified as an AS with sarcomatous overgrowth (ASSO). AS represents 5–10% of all US and ASSO is seen in 8–54% of AS [41, 42]. Recurrences are usually composed exclusively of mesenchymal elements and occur in 15–25% of AS or 45–70% of ASSO [1, 27]. Five-year survival for patients with AS in early-stage disease is 79% decreasing to 48% in patients with stage III disease. Mortality in patients with AS is 10–25% while in patients with ASSO can rise to 75%. Thus, patients with tumors exhibiting sarcomatous overgrowth have the poorest outcomes [1].
7.3 Clinical Presentation of Peritoneal Sarcomatosis
The mean age of presentation for US is 50 years, but it may arise at any age. Clinicians may encounter PS that has developed several years after hysterectomy or uterine surgeries for benign conditions. Frequently, retrospective reevaluation of pathology reports reveals sarcoma. Cases of PS after laparoscopic or robotic access with morcellation or slicing of uterine fibroids have been reported [10, 11, 43]. Considering the high risk of occult US in women over 50 years, caution should be used when minimally invasive surgery is performed [44]. Currently this treatment modality is under moratorium.
Patients usually experience symptoms that are nonspecific and that vary in intensity secondary to pelvic involvement. As patients become symptomatic, it is due to either tumor invasion into the adjacent organs (urinary, rectal, or vascular symptoms) or mass effect in the peritoneal cavity, which is often described as early satiety, bloating, or unintentional weight loss [21]. The problem of late diagnosis may be explained by the fact that the tumor has a lot of space to grow before any symptoms occur.
7.4 Management
In patients where a complete resection may be feasible, the first-line treatment for US is surgery, whether the disease is limited to the uterus or with metastatic spread [45].
7.4.1 Systemic Chemotherapy
Local recurrence or metastatic disease from US is a challenging condition to treat. There are a limited number of studies that evaluate the role of the different treatment modalities available today; however, palliative surgery and systemic chemotherapy are the most commonly used treatment, which may also be the only treatment available for patients with locally advanced, recurrent, metastatic, or inoperable initial presentation of the tumor. The median OS of patients with advanced or metastatic US is less than a year, and the median PFS is only 4 months for patients treated with systemic chemotherapy and CRS [46]. In a phase III study, Reed et al. have shown that adjuvant chemotherapy in patients with LMS did not significantly improve OS [47].
The National Comprehensive Cancer Network (NCCN) guidelines recommend either a single agent or a combination of two cytotoxic agents for systemic management of US based on the consensus of acceptable approaches to treatment by gynecologic and medical oncologists [32] (Table 7.1).
Table 7.1
Systemic chemotherapy for uterine sarcoma
Single-agent options | Combination regimens |
|---|---|
Dacarbazine | Docetaxel/gemcitabine |
Docetaxel | Doxorubicin/dacarbazine |
Doxorubicin | Doxorubicin/ifosfamide |
Epirubicin | Gemcitabine/dacarbazine |
Eribulin | Gemcitabine/vinorelbine |
Gemcitabine | |
Ifosfamide | |
Liposomal doxorubicin | |
Pazopanib | |
Temozolomide | |
Trabectedin | |
Vinorelbine |
Historically, PS and advanced US with metastases have had very limited response to systemic chemotherapy. Patients who received anthracyclines and ifosfamide (i.e., doxorubicin/ifosfamide) had a median survival of 1 year [48]. Gemcitabine with or without docetaxel has been used for palliative treatment. Demetri et al. demonstrated the effectiveness of trabectedin in patients with advanced LMS, with median OS of 12 months in patients with metastatic LMS [49].
Pautier et al. showed significant improvement of PFS at 3 years (55%) in patients with LMS treated with doxorubicin/ifosfamide/cisplatin and subsequent radiotherapy, although, this treatment had high toxicity rates [50]. Similar PFS (57%) with lesser toxicity was obtained in patients treated with docetaxel/gemcitabine followed by doxorubicin. This combination also has the highest response rate with median OS of 16 months as a first-line option in patients with metastatic US and 15 months as a second-line modality [20, 51, 52].
Different chemotherapeutic agents have been studied in order to find a monotherapeutic regimens or combinations of drugs for systemic chemotherapy in patients with advanced US. Complete response (CR) varied between 2% and 9% and partial response (PR) in the range of 13–44%. CR+PR have been found in 10–53% patients with OS and PFS between 12–26 months and 4–7 months, respectively (Table 7.2).
Table 7.2
Chemotherapy agents in metastatic uterine sarcoma with response rates and overall survival
Author | Chemotherapy agent | No. for analysis | CR | PR | CR+PR rate (95% confidence interval) | Median OS time (mos) | Median PFS (mos) |
|---|---|---|---|---|---|---|---|
Hensley et al. [19] | Gemcitabine/docetaxel | 34 | 9% | 44% | 53% (35–70) | 17.9 | 5.6 (4.3–9.9) |
Hensley et al. [20] | Gemcitabine/docetaxel (1st line) | 42 | 5% | 31% | 36% | 16.1 (4–41.3) | 4.4 (0.4–37) |
Hensley et al. [51] | Gemcitabine/docetaxel (2st line) | 48 | 6% | 21% | 27% | 14.7 (0.8–50.9) | 6.7 (0.7–27) |
Omura et al. [53] | Doxorubicin | 28 | NR | NR | 25% (9–41) | 12.1 | NR |
Look et al. [54] | Gemcitabine | 42 | 2% | 19% | 21% (7–31) | NR | NR |
Sutton et al. [55] | Liposomal doxorubicin | 32 | 3% | 13% | 16% | NR | NR |
Monk et al. [56] | Trabectedin | 20 | NR | 10% | 10% | 26.1 | 5.8 |
Eribulin, a microtubule binder which inhibits mitotic activity, shows better results than trabectedin as a single-agent modality in LMS patients with median OS of 14 months, while dacarbazine has had a significantly lower median OS of 12, (p < 0.05) [57]. Trabectedin was not included by the NCCN, since it was only approved in October 2015 as therapy for LMS.
Hormonal treatment has been used in combination with chemotherapy and might be beneficial in some patients. US may express estrogen and progesterone receptors (ER/PR) and, therefore, a target for hormonal therapy with drugs such as medroxyprogesterone acetate, megestrol, aromatase inhibitors, and gonadotropin-releasing hormone (GnRH) analogs [58]. Clinicians should bear in mind that the percentage of ER/PR positive tumors may vary among different histopathological types. It has been shown that among stromal sarcomas, 83% of LG-ESS express ER/PR, while only 10% of USS with nuclear uniformity express ER/PR [59]. ER/PR expression may be completely absent in USS with nuclear polymorphism, while LMS expression ranges between 30% and 80% [60, 61]. Patients with ER-negative sarcomas have demonstrated poor OS (median OS of 16 months) when compared to ER-positive sarcomas (median OS of 36 months), with ER positivity being an independent predictor of improved OS [6, 58].
7.4.2 Radiotherapy
Various authors reported controversial results regarding radiotherapy in patients with US. Although radiotherapy has shown some efficacy in patients with uterine carcinosarcoma, there is no significant improvement of OS in patients after adjuvant radiotherapy in ESS, since tumor relapse of the tumor is predominantly distant [62]. In addition, LMS histopathological subtype does not respond well to this treatment modality [47]. Additionally, radiotherapy did not improve local or distant recurrence rates; however, local pelvic radiation and brachytherapy may be beneficial in patients with cervical stromal involvement [63]. Meanwhile, Weitmann et al. have shown that radiotherapy in combination with surgical treatment may improve the OS (81% in 5 years) and provide local control in patients with ESS histopathological subtype of US [63].
While the overall role of radiotherapy is very limited, it may be useful in case of symptomatic recurrence or metastasis as a palliative treatment, when other treatment modalities are not feasible or may not improve patient quality of life [63].
7.4.3 Palliative Surgery
US with PS has a wide variety of clinical presentations. As with most malignancies, prognosis is better when detected early. Even with an aggressive surgical approach, including tumor debulking with negative margins and systemic chemotherapy, recurrence with locally advanced tumor may occur. These patients are the most difficult to manage, and often, the only treatment modality is a palliative approach [21].
The goal of palliative management is to alleviate symptoms, which in turn may improve the quality of life in suffering patients. Surgery is performed without curative intent; however, even palliative debulking of tumor may prolong life and improve quality of life. Additionally, surgery in incurable patients, may prevent development of bowel obstruction, respiratory difficulty, or ureteral and vena cava compression syndromes [21].
Patients with advanced, incurable malignancy should be considered candidates for palliative surgical treatment if resection, with or without radiation therapy, can be carried out with limited morbidity. Even partial resection of tumor with retroperitoneal extension can improve symptoms in up to 75% of patients [64].
7.4.4 CRS/HIPEC in Patients with PS from US
7.4.4.1 Introduction
Systemic chemotherapy, radiation therapy, and surgery have had minimal success in the management of patients with PS from US. Median OS using these modalities is between 15 and 29 months, supporting the necessity to develop new therapeutic approaches.
Patients with peritoneal disease from gastrointestinal or gynecological epithelial cancers (peritoneal carcinomatosis) have demonstrated improved outcomes following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy [65–75]. The rational of this dual approach is based on the assumption that surgery alone may not provide adequate local disease control; therefore, a complete cytoreduction, to remove all gross disease and reduce it to microscopic levels, coupled with delivery of hyperthermic intraperitoneal chemotherapy to eradicate microscopic tumor cells is required [76].
7.4.5 Previous Studies
The literature on CRS/HIPEC in patients with PS from US is limited. Several studies have evaluated this modality in patients with PS, but, due to the rarity of the disease, most studies consist of a combination of histopathological types. This tumor heterogeneity should be kept in mind, since tumor aggressiveness and biological behavior differ and will introduce bias when the overall outcomes are analyzed (Table 7.3).
Table 7.3
Clinical outcomes of CRS/HIPEC for peritoneal sarcomatosis from uterine sarcoma
Author/publication date (ref) | N | US n (%) | Histopathological subtypes | Clinical presentation | Treatment modalities and HIPEC/EPIC chemotherapy | CC 0–1 n (%) | Recurrence n (%) | Median OS (mos) | 5-year survival | Morbidity/mortality n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
Berthet et al./1999 [77] | 43 | 4 (9) | LMS (22), LPS (9), FBS (4), SRCS (4), SCC (2), Schw (1), HPC (1) | Recurrent (43) | -CRS/HIPEC: CDDP/DOX (3) -CRS/HIPEC + EPIC: CDDP + DOX (13) –CRS + EPIC: CDDP/DOX (7) or DOX (7) – CRS (13) | 27 (63) | NR | 20 | 30% CRS/HIPEC or EPIC: 39% CRS alone: 0% | 8 (19)/3 (7) |
Eilber et al./1999 [78] | 54 | 14 (26) | US [LMS:14], GIST (33), LPS (4), SCS (1), HS (1), OsS (1) | Recurrent (54) | -CRS/EPIC (No HIPEC): DHAD | 54 (100) | 8 (15) peritoneal 18 (33) peritoneal and liver 19 (35) liver | NR | 31%a Stage I: 46% Stage II: 5% | 5 (9)/0 (0) |
Rossi et al./2004 [23] | 60 | 12 (20) | US [LMS:8, ESS:4], GIST (14), RPS [LPS:20, MFH:6, Schw:4, FBS:2, SRCS:2] | Primary (29) Recurrent (31) | -CRS/HIPEC: CDDP/DOX | 60 (100)b | 27 (45) peritoneal 15 (25) peritoneal and distant | 34 | NR | 20 (33)/0 (0) |
Kusamura et al./2004 [22] | 10 | 10 (100) | US [LMS:8, ESS:1, AS:1] | Primary (2) Recurrent (8) | -CRS/HIPEC: CDDP/MMC (2) CDDP/DOX (8) | 9 (90) | 3 (30) peritoneal 3 (30) peritoneal and distant | NR | 65% | 0 (0)/0 (0) |
Baratti et al./2010 [13] | 37 | 11 (30) | US [LMS: 10, ESS: 1], RPLS (13), GIST (8), OSTS [SRCS: 3, MFS: 1, LMS: 1] | Primary (10) Recurrent (27) | -CRS/HIPEC: CDDP/DOX CDDP/MMC | 31 (84) | 16 (43) peritoneal 5 (14) distant | 26 | 24% | 8 (22)/1 (3) |
Sugarbaker et al./2016 [11] | 6 | 6 (100) | US [LMS: 6] | Recurrent (6) | -CRS/HIPEC/EPIC: HIPEC: CDDP/DOX + IFO (IV) (6) EPIC: PTX (5) | 6 (100) | 3 (50) distant | NR | NR | 2 (33)/0 (0) |
Sardi et al./Unpublishedc | 7 | 7 (100) | US [LMS:4, AS:2, ESS:1] | Primary (1) Recurrent (6) | -CRS/HIPEC: CDDP/DOX L-PAM | 7 (100) | 3 (43) peritoneal 1 (14) distant | Not reached | 57% | 0 (0)/0 (0) |
One of the first studies that used CRS/HIPEC in patients with PS was carried out by Berthet et al. [77], who studied 43 patients with recurrent abdominopelvic sarcoma from different origins between 1989 and 1996. The histological subtypes varied with just four patients with tumors arising from the uterus. Intraperitoneal chemotherapy was performed in 30 patients with complete cytoreduction: 16 HIPEC with cisplatin/doxorubicin (n = 3) or cisplatin followed by early postoperative intraperitoneal chemotherapy (EPIC) with doxorubicin (n = 13) and 14 EPIC with cisplatin/doxorubicin (n = 7) or doxorubicin (n = 7) alone. The remaining 13 patients were managed with cytoreductive surgery (n = 13) without any chemotherapy. The median survival was 20 months for all patients. Those with a complete cytoreduction plus intraperitoneal chemotherapy demonstrated an improved 5-year survival when compared to patients who received surgery alone (39% versus 0%, respectively).
Eilber et al. [78] published another prospective study in 54 patients with recurrent abdominal sarcoma enrolled between 1990 and 1997. Fourteen patients had primary US. Patients were divided by stage II (n = 35) or stage III (n = 19) according to the presence of hepatic metastases at the time of presentation. All patients underwent CRS/EPIC with mitoxantrone (20 mg/m2). Forty-five patients (83%) recurred with a mean interval to recurrence of 11 months (median, 9 months, and range, 1–53 months). Peritoneal and liver recurrence rates were 48% and 69%, respectively. Overall 5-year survival was 31%, with the best results in the stage II group compared with the stage III group, 46% and 5%, respectively. Although the study did not describe how many patients with primary US were included in each group, they concluded that CRS/EPIC with mitoxantrone significantly lowered the rate of peritoneal recurrence and provides benefit for patients with disease limited to the peritoneum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree