Introduction
Osteoporosis is a skeletal disorder, characterized by compromised bone strength, predisposing a person to an increased risk of fracture. The major fragility fractures are those of the forearm, vertebral body and hip, but fractures of the humerus, pelvis and tibia are not uncommon in patients with osteoporosis. Fragility fractures are a major cause of excess mortality, substantial morbidity and health and social service expenditure in older people.1 It is therefore important that effective strategies are developed and implemented to prevent these fractures.
Epidemiology of Osteoporosis and Fractures
The World Health Organization (WHO) has quantitatively defined osteoporosis as a bone mineral density (BMD) 2.5 standard deviations or more below the mean value for young adults (T-Score ≤−2.5).2 The prevalence of osteoporosis at the hip increases in women from 8% in the seventh decade of life to 47.5% in the ninth decade. The incidence of fragility fractures also increases with advancing age, but is higher in women than men. The majority of these fractures occur in people above the age of 75 years, with a three- to fourfold higher rate in institutionalized older people than community-dwelling individuals of the same age. The lifetime risk of fragility fractures for a 50-year-old woman in the UK is 53.2%, compared with 20.7% for a 50-year-old man.1
Although forearm fractures may lead to deformity of the wrist and the development of osteoarthritis and the complex regional pain syndrome, there is no evidence of an increase in mortality. Nevertheless, forearm fractures are associated with an increased risk of vertebral and hip fractures in both men and women, so provide an opportunity for consideration of secondary prevention.
Only a third of vertebral fractures come to medical attention, but symptomatic vertebral fractures have a major impact on a patient’s quality of life (QOL), because of back pain, loss of height and kyphosis. Vertebral fractures may also result in loss of energy, emotional problems, sleep disturbance, social isolation and reduced mobility. Studies suggest that the impairment of QOL increases with the number of vertebral fractures. The magnitude of the problems encountered by patients with symptomatic vertebral fractures is demonstrated by the fact that they visit their GP 14 times more often than control subjects in the year following fracture. Vertebral fracture is associated with an increased risk of further vertebral fractures, which may be as high as 20% in the following year. There is also an increased risk of hip and other non-vertebral fractures. Vertebral fractures are associated with an excess mortality of 17–20%, which is likely to be due to coexisting conditions associated with osteoporosis, rather than the fracture itself.1, 3
Hip fractures are the most important fractures in older people, as they cause greater morbidity, higher mortality and more expenditure than all the other fragility fractures combined. Between 25% and 50% of patients become more immobile and dependent after hip fracture, but this is particularly apparent in men and women above the age of 75 years, those with a poor clinical outcome and those who were already dependent before fracture. The excess mortality following hip fractures has been reported to be about 17% over five years, but most deaths occur within six months, suggesting that this is due to complications arising from the fracture and subsequent surgery. Mortality after hip fracture also increases with the number of comorbid conditions. A number of studies show a higher mortality after hip fracture in men than women, but the reason for this is still unclear.1 It has been estimated that fragility fractures are associated with health and social service costs of 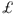 2.3 billion in the UK, almost 90% of which is due tox hip fractures. It has estimated that the average cost of hip fracture in the UK is
2.3 billion in the UK, almost 90% of which is due tox hip fractures. It has estimated that the average cost of hip fracture in the UK is 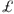 12 000, of which
12 000, of which 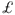 4800 is due to the cost of acute hospital care. About 40% of all patients with hip fracture have experienced a prior fragility fracture, potentially representing a lost opportunity for secondary prevention. There is also rapid bone loss after hip fractures and an increased risk of fracture of the contralateral hip and at other sites.
4800 is due to the cost of acute hospital care. About 40% of all patients with hip fracture have experienced a prior fragility fracture, potentially representing a lost opportunity for secondary prevention. There is also rapid bone loss after hip fractures and an increased risk of fracture of the contralateral hip and at other sites.
Bone Remodelling Throughout Life
Bone is a living tissue which constantly remodels throughout life, allowing the skeleton to grow during childhood, respond to the mechanical forces placed on it and repair damage due to structural fatigue or fracture. The three major bone cells involved in bone remodelling are osteoclasts, osteoblasts and osteocytes. Osteoclasts are multinucleate cells derived from macrophage-monocyte precursors which resorb bone. Osteoblasts are derived from fibroblast precursors and produce bone matrix or osteoid, which is then subsequently mineralized. Osteocytes are mature osteoblast trapped within calcified bone, which have long interconnecting dendritic processes, and which may serve as mechano-sensory receptors.1
Recent research has highlighted the major role of the receptor activator of nuclear factor kappa B (RANK) and RANK ligand (RANKL) system in the regulation of bone remodelling. RANKL is produced by osteoblasts and attaches to RANK on the surface of osteoclasts and osteoclast precursors, leading to osteoclast differentiation and proliferation. The action of RANKL on RANK is blocked by osteoprotegerin (OPG), a decoy receptor produced by osteoblasts and marrow stromal cells. It is now apparent that the beneficial effects of osteoporosis treatments may be mediated in part by changes in the RANK, RANKL and OPG system.4
Another regulator of bone turnover is sclerostin, which is produced by osteocyte, under the control of the SOST gene. Sclerostin binds to low density lipoprotein receptor-related protein 5 (LRP5) and inhibits the Wnt signalling pathway, leading to reduced bone formation. Mutations associated with loss of function of the SOST gene lead to sclerosteosis, characterized by increased bone formation, whereas mutations of the LRP5 gene cause the osteoporosis pseudoglioma syndrome.5
Pathogenesis of Osteoporosis and Fractures
BMD at any age is determined by the peak bone mass achieved at maturity, the age at which bone loss starts and the rate at which it progresses. Genetic factors account for up to 80% of the variance in peak bone mass, with the remainder being due to environmental factors, exercise, diet and age at puberty. Bone loss starts between the ages of 35 and 45 in both sexes, but this is accelerated in the decade after the menopause in women. Bone loss then continues until the end of life in men and women, aggravated by factors such as physical inactivity, smoking, alcohol consumption and vitamin D insufficiency, and secondary hyperparathyroidism. There are also a number of causes of secondary osteoporosis, including oral glucocorticoid therapy, male hypogonadism, hyperthyroidism, primary hyperparathyroidism and the use of anti-epileptic drugs.6
The risk of fractures is determined by skeletal and non-skeletal risk factors. There is an inverse relationship between bone density and fracture risk, with a two- to threefold increase in fracture incidence for each standard deviation reduction in BMD. The risk of fracture is also determined by other skeletal risk factors, such as bone turnover, cortical and trabecular bone architecture, skeletal geometry and the degree of mineralization of the skeleton. Non-skeletal risk factors for fracture include postural instability, impaired neuromuscular function, physical and mental frailty, and reduced fat and muscle bulk around the hip.6
Up to 30% of women and 55% of men with symptomatic vertebral fractures have an underlying cause of secondary osteoporosis, such as oral glucocorticoids, anti-epileptic medication, male hypogonadism, hyperthyroidism, alcohol abuse and myeloma. Risk factors for hip fracture include causes of secondary osteoporosis and conditions associated with falls, such as stroke, Parkinson’s disease, dementia and visual impairment.1, 6
Diagnosis of Osteoporosis
Osteoporosis may be diagnosed by performing BMD measurements at the lumbar spine, total hip and femoral neck using dual energy X-ray absorptiometry (DXA). The WHO definition of osteoporosis (T-Score ≤−2.5) was initially developed for epidemiological studies to assess the prevalence of the condition in different populations, but has increasingly been used as a threshold for diagnosis and therapeutic intervention. Although DXA measurements are generally accurate and precise, lumbar spine BMD may be spuriously elevated in the presence of vertebral fractures, degenerative changes and aortic calcification. Furthermore, only 50% of people with fragility fractures have osteoporosis on DXA scanning, suggesting that other skeletal and non-skeletal risk factors are important in determining fracture risk.
Fracture Risk Assessment
The WHO has developed a fracture risk assessment tool (FRAX®), which estimates the 10-year risk of fractures of the major fragility fractures (forearm, humerus, spine and hip) and of hip fracture in particular.7 Country-specific algorithms use age, gender, weight, height and the presence or absence of appropriately weighted risk factors, with or without femoral neck BMD measurements, to estimate fracture risk. The clinical risk factors for fracture used in FRAX®, which are at least in part independent of BMD, comprise low body mass index (BMI), prior fracture after age of 50 years, parental history of hip fracture, current smoking, oral steroid therapy, alcohol intake >2 units/day and chronic conditions associated with bone loss such as rheumatoid arthritis.7 Other fracture risk assessment tools are being developed, with a view to identifying people at the highest risk of treatment, in whom to target therapeutic intervention.
Guidance has been developed in the USA, UK and in other countries on the level of fracture risk at which treatment should be considered. This will depend not only on the health economy of the country and the cost of the available therapeutic options, but also individual patient factors such as age and the presence of other comorbid conditions. Most clinical trials of treatments to prevent fractures have recruited participants on the basis of documented osteoporosis or the presence of vertebral fractures. Some clinicians are therefore reluctant to use these treatments at patients at high risk of fracture who have a BMD T-Score >−2.5, as there is no definite evidence that they will prevent fractures in this situation.
Investigation
In patients with documented osteoporosis, underlying causes of bone loss should be identified by careful history, physical examination and appropriate investigation (Table 90.1), particularly when the BMD is lower than expected for age (Z-Score <2.0). These investigations should also be considered in patients with fragility fractures, as specific treatment of underlying conditions such as hyperthyroidism, hypogonadism and primary hyperparathyroidism may increase BMD by up to 15%.1, 6 Investigations for hypogonadism in men may be less appropriate in older men, where the adverse effects of testosterone replacement on the prostate may outweigh the potential benefits. Serum 25-hydroxyvitamin D (25OHD) and parathyroid hormone (PTH) measurements may show vitamin D insufficiency and secondary hyperparathyroidism, but these measurements are probably unnecessary if calcium and vitamin D supplementation is planned. Nevertheless, serum 25OHD and PTH measurements should be considered in patients with possible vitamin D deficiency osteomalacia, which is particularly likely in housebound patients or those with previous gastric resection, malabsorption or the long-term use of anti-epileptic drugs. In patients with severe unexplained osteoporosis, low BMI or anaemia, investigations to exclude a diagnosis of coeliac disease should be performed, particularly if there are symptoms of possible malabsorption.
Table 90.1 Investigations in patients with fragility fractures or low BMD.
| Investigation | Finding | Possible cause |
| Full blood count | Anaemia Macrocytosis | Malignancy or malabsorption Alcohol abuse or malabsorption |
| ESR and CRP | Raised inflammatory markers | Malignancy |
| Biochemical profile | Hypercalcaemia Abnormal liver function tests Persistently high AP | Hyperparathyroidism or malignancy Alcohol abuse or liver disease Skeletal metastases |
| Thyroid function tests | Suppressed TSH; high T4 or T3 | Hyperthyroidism |
| Testosterone, SHBG, LH, FSH (men) | Low total testosterone or calculated free testosterone with abnormal gonadotrophins | Hypogonadism |
| PSA (men with vertebral fractures) Serum and urine electrophoresis (Patients with vertebral fractures) Serum 25OHD and PTH | Raised PSA Paraprotein band Low 25OHD and raised PTH | Metastatic prostate cancer Myeloma Vitamin D insufficiency and secondary hyperparathyroidism. |









