system. ORN is also preferentially conducted for patients with bulky tumors, or in patients with nodal and metastatic involvement (i.e., “cytoreductive” ORN). In terms of indications for open versus laparoscopic technique selection, this is primarily guided by patient and surgeon preference and expertise as well as the expected level of technical difficulty. In many patients with large tumors, prior abdominal surgery, complex or anomalous vasculature, or vascular involvement, the open approach is often preferable to limit OR time and to maximize safety of the operation.
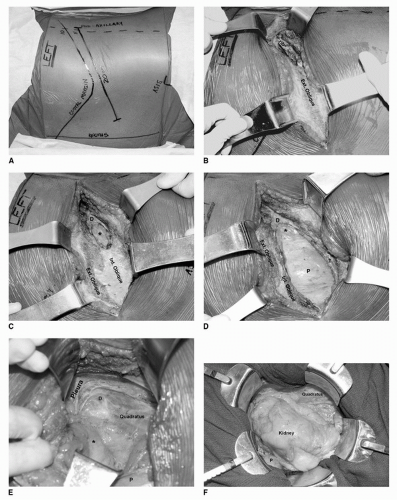
multiple large-caliber parasitic vessels. By remaining in the correct plane outside of Gerota’s fascia, most of these vessels can be avoided. Lumbar veins drain into the vena cava at each vertebral level and can be avulsed when too much traction is placed on the vena cava. In addition, there are typically large lumbar veins that can drain directly into the renal vein or vena cava at the level of the renal veins, particularly on the left side. The renal veins should be mobilized sufficiently prior to ligation to allow for identification of these lumbar vessels posteriorly. Other sources of possible hemorrhage are the gonadal and adrenal veins. The gonadal veins on either side can be ligated early in the procedure to prevent avulsion during kidney retraction and hilar dissection. Care should be taken on the superior aspect of the left renal vein to avoid bleeding from the adrenal vein. Likewise, care should be taken near the posterior infrahepatic IVC to avoid the right adrenal vein. Pinhole or subcentimeter venotomies can often be controlled by placement of figure-of-eight 5-0 vascular sutures. Larger lacerations can be controlled with a series of Allis clamps followed by sewing of the caval wall with a running 5-0 vascular suture. In cases of large venotomies, compression should be applied on the area of hemorrhage, and the IVC and other venous tributaries should be controlled with atraumatic vascular clamps so that formal venous reconstruction can be conducted.
injury, bowel obstruction, or pneumothorax. Three patients required splenectomy for splenic injuries. Median hospital stay duration was 6 days. Length of stay and complication rates were similar in other studies; however, reporting of complications was not standardized (17,18). A recent meta-analysis for the management of clinical T1 renal masses found that ORN had a urological complication rate of 1.3%, which was significantly lower than all other treatment modalities including laparoscopic RN, open or laparoscopic PN, and ablative therapies (11). Patients with T2 disease or greater, high operative blood loss, and increased length of stay had an increased risk of perioperative DVT and PE (19). The role of heparin DVT prophylaxis in preventing venous thromboembolic events remains to be studied in patients undergoing RN or PN.
TABLE 44A.1 CONTEMPORARY RESULTS FOR CANCER-SPECIFIC AND OVERALL SURVIVAL FOR RADICAL NEPHRECTOMY FOR LOCALIZED RCC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
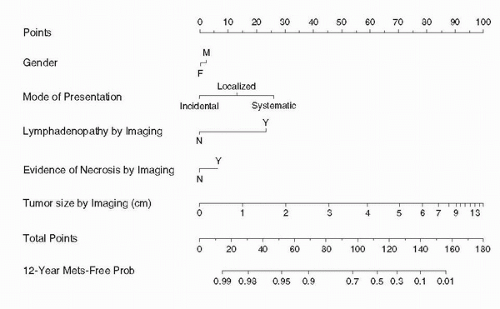
mainstays in prediction of RCC recurrence after ORN. Compared to patients with T1 tumors, those with T2, T3, and T4 tumors have a relative risk of disease progression of 2.3, 4.5, and 11.4, respectively. Compared to patients with Fuhrman grade 1 tumors, those with grade 3 and 4 histology have a relative risk of disease progression of 2.7 and 4.0, respectively (42). The greatest risk for recurrence for RCC occurs within the first 5 years following nephrectomy, although this remains one of the least predictable malignancies. For patients who recur after radical nephrectomy, 43% occur in the first year, 70% within 2 years, 80% within 3 years, and 93% within 5 years (23). Recurrence for T1 disease is reported to be 7% at a median time from diagnosis of 38 months, 26% for T2 disease at a median time of 32 months, and 39% for T3 disease at a median time of 17 months (43). Stage-based surveillance protocols stratify the intensity of surveillance based on the pathologic stage of the tumor (23,27,43,44,45), and reduce the costs of surveillance substantially by rationally reducing the frequency of follow-up CT scans. Radiologic surveillance for T1 and T2 tumors includes yearly history and physical examination for up to 5 years, with chest radiography and biochemical profile every 6 months to 1 year for up to 5 years. Abdominal imaging is not usually recommended for T1 tumors, but some authors recommend abdominal CT scans at 2 and 5 years. Surveillance for T3 and above is more rigorous, and involves clinical assessment and physical examination with biochemical profile and chest radiography every 6 months for up to 3 years, and yearly thereafter. Abdominal CT scan should be performed starting 6 to 12 months postoperatively, yearly for up to 3 years, and then every 2 years thereafter.
TABLE 44A.2 SELECTION OF VALIDATED POSTOPERATIVE PROGNOSTIC MODELS AND ALGORITHMS FOR RCC | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
of LRN for large tumors (8,9,10,11). The initial case series of LRN for tumors with renal vein involvement was reported in 2003, and with larger series now reported in the literature, renal vein involvement is no longer a contraindication for LRN (12,13,14). In our own experience, LRN for T3b tumors was associated with similar operative times, complication rate, and hospital stay when compared to tumors ≤T2 (unpublished data). Patients with more advanced disease, node positive or metastatic, are also candidates for LRN. While the role of lymphadenectomy in the surgical treatment of RCC can be debated (discussed elsewhere in the kidney cancer section), extensive lymphadenectomy can be performed laparoscopically with good outcomes and excellent node counts (15,16). Cytoreductive nephrectomy in patients with distant metastases is indicated in selected patients prior to systemic therapy. LRN in these patients may be preferable to minimize the time to convalescence and potentially the time to initiation of systemic therapy (17,18,19).
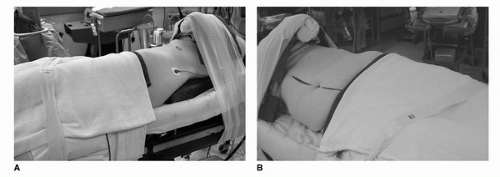
45 degrees. For retroperitoneal procedures, we place patients in the full flank position at 90 degrees. Proper padding of the patient along the entire length of the table is important along with adequate anterior and posterior support to maintain the decubitus position. In the full flank position, the table should be flexed 30 to 45 degrees to maximize the space between inferior costal margin and the superior iliac spine (very important in retroneoscopic procedures). The most vulnerable point involves positioning of the arms and the potential for brachial plexus injury. In the full 90-degree flank position, an axillary roll should be placed just caudal to the axilla (this is generally not necessary in the 45-degree modified flank position). The ipsilateral arm should be placed on a gel pad over the chest or an elevated padded arm rest depending on the degree of flank position. Care should be taken to avoid anterior flexion of the shoulder beyond 90 degrees. The contralateral arm should be placed on an arm board with proper ulnar padding. After securing the patient with 3 inch wide tape, the operating room table must be fully rotated prior to draping to ensure that the patient is stable in all positions. Finally, orogastric drainage, lower extremity sequential compression devices, and Foley catheter drainage are recommended.
TABLE 44B.1 RANDOMIZED CONTROLLED TRIALS TRANSPERITONEAL VERSUS RETROPERITONEAL NEPHRECTOMY | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 44B.2 TRIALS COMPARING CONVENTIONAL LAPAROSCOPIC VERSUS HAND-ASSISTED LAPAROSCOPIC NEPHRECTOMY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
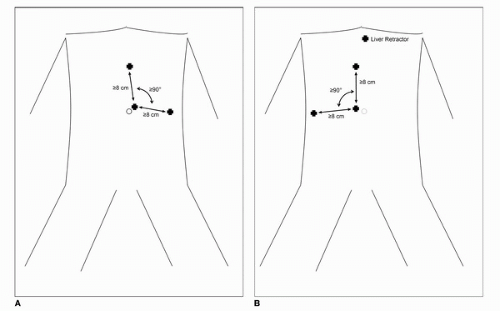
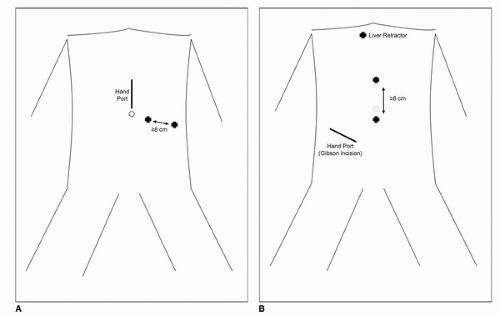
begin insufflation, two recent comprehensive reviews of the literature have failed to show a difference between the closed and direct (Hassan open) techniques with regard to prevention of major complications (28,29,30). In patients without prior umbilical incisions, we recommend transumbilical insertion of the Veress needle. After insertion, low-flow insufflation is begun to ensure initial pressures of <10 mm Hg. Once safe entry has been established, high-flow insufflation is begun to peak intra-abdominal pressure of 20 mm Hg followed by initial trocar placement. Stabilization of the abdominal wall may or may not be used during insertion of the Veress needle although routine elevation does not seem to prevent needle bowel injuries. In patients with prior abdominal surgery, the Veress needle can be inserted into any of the four quadrants furthest away from the site of prior surgery. In both the left and right upper quadrant, the needle should be inserted at least two finger breadths inferior to the costal margin in the midclavicular line. In the lower quadrants, the needle should be inserted three to four finger breadths medial to the anterior superior iliac spine. Although multiple maneuvers have been described to confirm safe intraperitoneal placement, such as, injection/aspiration of saline and the drop test, we do not employ these maneuvers routinely. Furthermore, none of these techniques have demonstrated efficacy in the literature more so than ensuring that intra-abdominal pressure on entry is <10 mm Hg. If after several attempts, safe intraperitoneal needle placement is not successful, the direct technique as described by Hasson can be performed (31). Once insufflation to 20 mm Hg is achieved, a blunt tip trocar (with or without the laparoscope) can be advanced into the abdomen. The remaining trocars are then placed under direct laparoscopic vision. For the vast majority of LRN, a three port (with additional 3-mm instrument for liver retraction on the right side) can be used. Fig. 44B.2A and B shows our typical port placements for left-and right-sided approach; however, multiple different configurations have been described in the literature. Additional trocars can be placed in the lower midline or in the subcostal space at the anterior axillary line as necessary for assistants. Once the trocars are in position, the table is fully rotated toward the contralateral side to maximize bowel displacement.
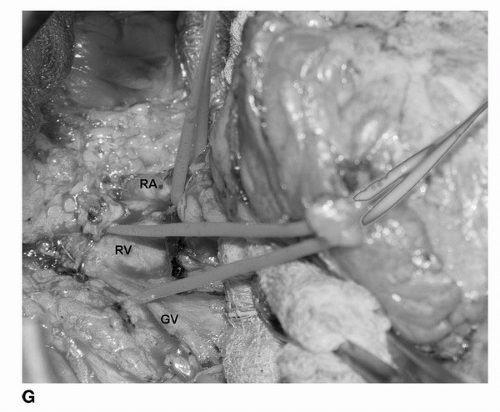
renal vein. It is during this dissection that the renal artery is encountered. We have found that the laparoscopic DeBakey instrument is especially useful during this dissection.
(30-40 pumps). The advantage of the commercially available device is that a laparoscope can be inserted via the dissector for direct visualization of the balloon dissection. This process is repeated in the cephalad direction to ensure adequate development of the retroperitoneal space. A blunt tip trocar (Covidien, Mansfield, Massachusetts) is then inserted to seal the incision site. The first working port is placed directly posterior to the middle port just lateral to the paraspinous muscles. Through this port, a blunt instrument is used to mobilize the peritoneum medially for placement of the second working trocar. Figure 44B.4 shows patient with trocars in place.
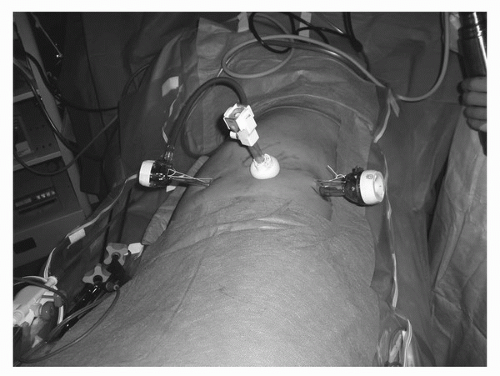 FIGURE 44B.4. Patient with trocars in place for retroneoscopic approach. Camera trocar is placed just off the tip of the 12th rib. |
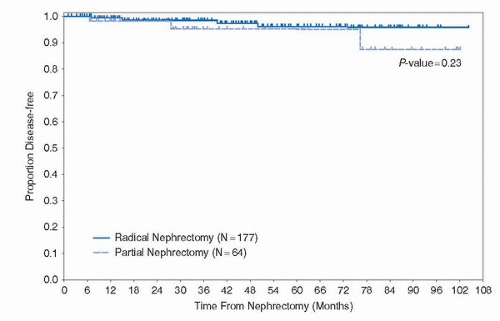
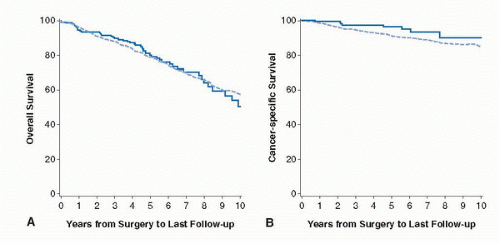
157 patients from five institutions undergoing LRN with mean follow-up of 19.2 months (with 51 patients with at least 24 months follow-up) (35). Overall, the authors reported at 91% 5-year actuarial disease-free survival for the entire cohort, 89% for clinical stage T2, and 86.9% for high-grade clinical stage T2 patients. With a modest complication rate of 9.6%, this report established the safety and short-term oncologic efficacy of LRN in the treatment of RCC. Since then, multiple single institution series have established similar long-term oncologic outcomes for LRN when compared to ORN (Table 44B.3). As such, in many academic centers, LRN has become the standard of care for surgical extirpation of RCC that is not amenable to nephron-sparing surgery. Contemporary series of LRN have focused on outcomes associated with high-risk tumors (Table 44B.4). Again, long-term follow-up demonstrates similar cancer-specific survival compared to historical controls of ORN. Recently, Berger et al. reported a multi-institutional series of LRN with follow-up of 11.2 years, by far the longest follow-up in the literature (36). With a mean age of 60 years and mean tumor size 5 cm, the authors reported an actual 10-year recurrence-free, cancer-specific, and overall survival of 86%, 92%, and 65%, respectively. These figures are comparable to established ORN series.
TABLE 44B.3 LONG-TERM OUTCOMES LAPAROSCOPIC VERSUS ORN | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 44B.4 EFFICACY OF LRN FOR HIGH-RISK TUMORS (≥PT2) | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
open surgery should be followed to minimize the risks. These principles include minimizing direct tumor handling, avoidance of tumor spillage in the operative field, placement of the tumor in an impermeable sac prior to extraction, and removal of all contaminated instrumentation including the surgeon’s gloves following extraction.
complications, while RN enjoyed increasing success especially when performed by urologists (1,2).
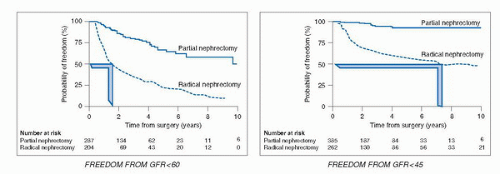
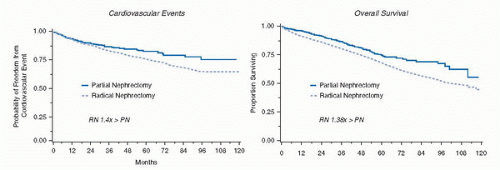
a major public health problem in the United States, and since 2003, it is considered an independent cardiovascular risk factor (44,45,46,47,48). An estimated 19 million adults in the United States have CKD and by the year 2030, 2 million will be in need of chronic dialysis or renal transplantation (49). Traditional risk factors for CKD include age >60, hypertension, diabetes, cardiovascular disease, and family history of renal disease, factors also common in the population of patients who develop RCTs.
elective kidney surgery. In addition, calculation of eGFR should be done using the following web link for the MDRD equation (http://www.nephron.com/MDRD_GFR.cgi) and in approximately 26% of patients, many of whom were previously unaware, CKD will be diagnosed (53) because of an eGFR < 60 mL/min/1.73 m2. If so, the PN would be classified as essential rather than elective. A risk stratification system, SCORED (screening for occult renal disease), was created to predict patients likely to develop CKD after kidney surgery. Patients with high SCORED values were most in need of kidney-sparing surgical approaches (60). A careful evaluation of outside imaging needs to be done. A surgeon should not operate on a renal mass without a noncontrast CT image to rule out the possibility of detecting macroscopic fat indicative of angiomyolipoma (7) and a contrast-enhanced study (MRI, CT, or renal perfusion/excretion scan) indicating bilateral renal function. Renal protocol CT imaging, which consists of three imaging sequences including precontrast, corticomedullary phase, and late nephrogenic excretory phase, provides a high degree of diagnostic accuracy in diagnosing RCTs (100% specificity, 95% sensitivity) (61) but cannot determine if the mass is benign or malignant. The degree to which a renal mass enhances is dependent on the CT scanner being utilized. For a single detector scanner, 10 HU was considered suspicious for a RCT, but with more modern multidetector scanners, “pseudo enhancement” of renal cysts secondary to volume averaging and beam hardening effects, particularly in smaller lesions, could lead to operation for a benign cyst. Enhancement of 10 to 20 HU in this setting may lead the radiologist to ask for further imaging often in the form of renal ultrasound with Doppler imaging to assess for vascular flow within the mass (62). Ultrasound can more fully characterize cystic lesions and serves as an effective template for intraoperative ultrasound, which can be highly effective in locating small subcortical renal tumors.
Nearness to collecting system or sinus, Anterior or posterior, and Location relative to the polar line) was developed as a means to document and describe surgical difficulty for a planned PN. Examples of scoring in the RENAL system are as follows; tumors that were 4 cm or less are given 1 point, 2 points for 4 to 7 cm, 3 points for >7 cm. Tumors that are 50% or more exophytic are assigned 1 point, tumors <50% exophytic are assigned 2 points, and tumors that are endophytic are assigned 3 points. As more points are accumulated, the degree of difficulty to perform PN increases. The authors used their system to score 50 consecutive PN and RN performed by open, laparoscopic, or robotic-assisted techniques and correlated the operative experience as the masses were deemed low (score 4-6), moderate (score 7-9), and high complexity (score 10-12). Few RN were performed by any technique for the low scored patients (64).
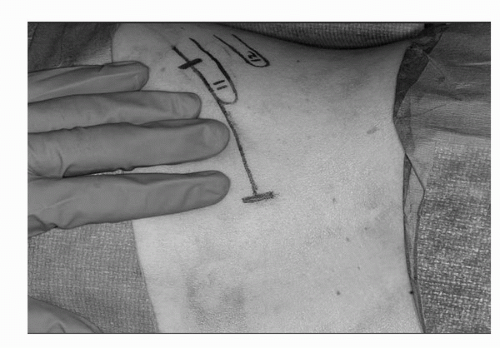 FIGURE 44C.6. Mini-flank surgical incision, approximately 8 to 10 cm, with incision above the 11th rib and in the space between the 10th rib. (Adapted from Diblasio CJ, Snyder ME, Russo P. Mini flank supra-eleventh incision for open partial or radical nephrectomy. BJU Int 2006;97:149-156.) (See color insert.) |
there is an intrarenal vein thrombus or tumor encroachment upon the renal collecting system (67) (Fig. 44C.8). Occasionally, a polar segmental artery may feed the exact tumor bearing area of the kidney. Ligation and division of this artery allows for a “regional ischemia” and precise resection with little damage to non-tumor-bearing kidney. When there is a completely intrarenal tumor without any evidence on the renal cortical surface, measurements from each pole of the involved kidney in millimeters are made using the preoperative CT scan with subsequent corresponding marks made on the renal cortex, the position of the endophytic tumor is then confirmed precisely with the intraoperative ultrasound. For a purely exophytic tumor or in a patient with significant underlying CKD, resection of the tumor without renal artery occlusion is carried out. For other patients with large, endophytic, or perihilar tumors who require renal artery occlusion, renoprotective measures including mannitol infusion (12.5 g/200 mL of normal saline) and ice slush are routinely used. It is no longer necessary to place the kidney in a plastic bag prior to ice slush placement since the small surgical mini-flank incision does not lead to patient hypothermia (Fig. 44C.9). In no cases of open PN is renal artery occlusion used without such renoprotective measures recommended (warm ischemia).
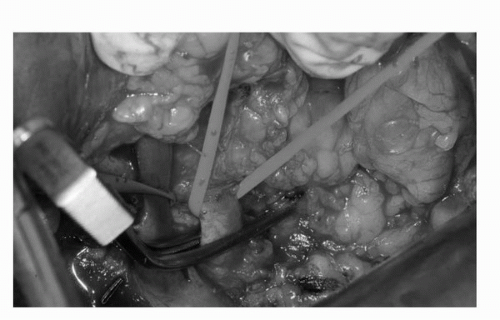 FIGURE 44C.7. The renal vein and renal artery (arteries) are carefully dissected from surrounding lymphatic soft tissues and identified by red (artery) and blue (vein) vessel loops. During cold ischemia, a bulldog vascular clamp is applied to the renal artery. (See color insert.) |
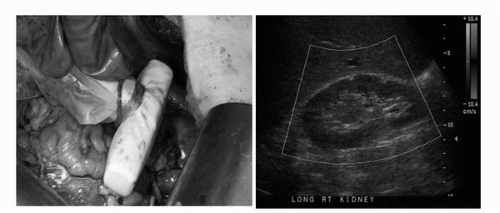 FIGURE 44C.8. Intraoperative ultrasound is routinely performed to locate endophytic tumors, assess kidney for satellite tumors, collecting system invasion, or branched renal vein thrombus invasion. (See color insert.) |
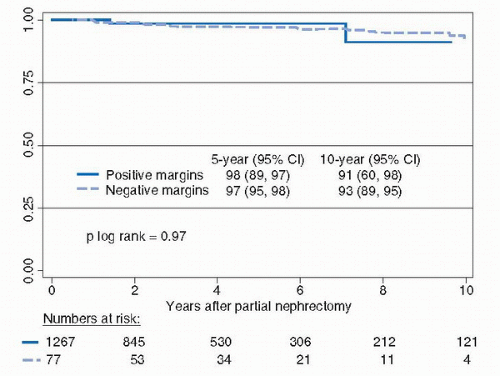
carefully inspected to be certain that the lesion is intact and that there is a complete covering layer of kidney and soft tissue. The deep tumor surgical margin is marked with a silk suture to orient the specimen, which is then delivered to the pathology department fresh and in sterile condition. Frozen section of the deep margin and specimen can provide immediate reassurance to the surgeon and the family, but a final pathological diagnosis of the renal cortical tumor may not be available by frozen section due to the need to perform immunohistochemical or cytogenetic analysis. With the aide of 2.5× loupe magnification, defects in collecting system and small vessels are easily identified and closed/ligated using 3-0 or 4-0 absorbable suture with special care taken to separately repair veins and arteries and avoid large bulky deep bites into the renal sinus, which could cause an iatrogenic a-v fistula or pseudoaneurysm.
 FIGURE 44C.9. Renoprotective ice slush is applied to the kidney with careful time kept to accurately record the cold ischemia time. A protective bag is no longer employed since the mini-flank incision is small and patients do not become hypothermic during the resection. (See color insert.) |
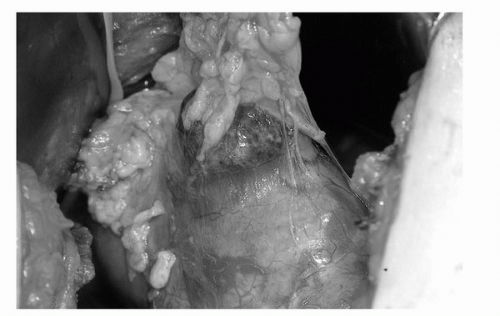 FIGURE 44C.10. Exophytic tumor with surrounding fat intact. A 1-cm margin of normal renal cortex is scored using electrocautery prior to commencing the PN. (See color insert.) |
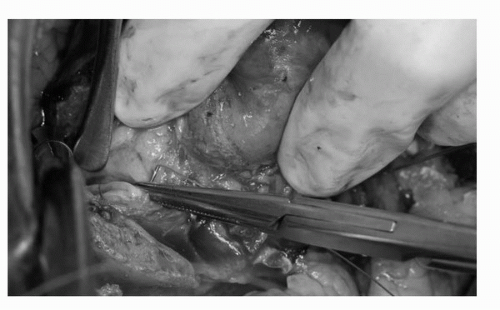 FIGURE 44C.11. Repairs are made in the collecting system and rents in renal sinus arteries and veins are repaired with absorbable 3-0 and 4-0 sutures. (See color insert.) |
period, at 1 month, and at 12 months postoperatively. Patients with a solitary kidney had a baseline eGFR 30% lower than patients undergoing elective PN. Median cold ischemia time was 35 minutes in the elective PN group and 31 minutes for patients with a solitary kidney. Patients with a solitary kidney experienced a greater decline in eGFR compared to elective PN patients in the early postoperative period (30% vs. 16%), at 1 month (15% vs. 13%), and at 12 months (32% vs. 12%). Upon multivariate analysis, duration of cold ischemia and intraoperative blood loss, both likely surrogates for difficult operations, was significantly associated with early changes in eGFR but by 12 months age was the only significant predictor of eGFR decrease in patients undergoing elective PN. Although this study does not answer critical questions concerning long-term renal damage in either group of patients, it is clear that those patients with tumor in a solitary kidney and the elderly are more vulnerable to renal damage after PN (74). Investigators combined the Mayo and Cleveland Clinic 18-year experience with 458 patients undergoing PN in a solitary kidney and warm ischemia. No ischemia was used in 96 patients (21%) while 362 (79%) had a median of 21 minutes of warm ischemia. Warm ischemia patients were significantly more likely to develop acute renal failure and an eGFR of <15 mL/min/1.73 m2 and stage 4 CKD during a 3-year follow-up when compared to patients whose resection was completed without hilar clamping (75). Another multi-institutional study retrospectively evaluated 537 patients with a solitary kidney and baseline CKD indicated that the rate of chronic renal insufficiency or more severe CKD (defined as serum creatinine > 2.0 ng/mL) was 26% when no renal artery occlusion was used, 30% after warm ischemia, and 41% after cold ischemia. In this study the cold ischemia may have been selectively utilized in more challenging cases. The authors felt a cutoff of 20 minutes of warm ischemia could decrease the risk of severe CKD in this highly susceptible patient population (76).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree