© Springer International Publishing Switzerland 2016
Stuart Silverman and Bo Abrahamsen (eds.)The Duration and Safety of Osteoporosis Treatment10.1007/978-3-319-23639-1_1616. Management of Drug Holidays
(1)
Service de Rhumatologie, Hôpital Cochin, 27 rue du Faubourg Saint Jacques, 75014 Paris, France
(2)
INSERM U1153, Paris-Descartes University, Paris, France
Keywords
Drug holidayOsteoporosisFractureBisphosphonateAtypical femoral fractureBone mineral densityBiomarkers of bone remodelingDiscontinuation of treatmentSummary
Bisphosphonates are effective in reducing osteoporotic fracture risk.
Concerns were raised recently about long-term bone retention of bisphosphonates.
The rationale for a drug holiday (temporary discontinuation) is the high affinity of bisphosphonates for bone.
A drug holiday could be considered after 3–5 years of treatment, providing that the patient is not at persistent high risk.
There is no evidence-based data about benefit and risks of such drug holidays.
Introduction
A drug holiday is a temporary discontinuation of a drug. In the context of osteoporosis , this concept has been proposed insistently because of safety concerns about long-term administration of bisphosphonates . The incidence of these bisphosphonate-related adverse events is low, but their perception is high. The rationale for this concept is the property of bisphosphonates to be accumulated in bone over time and released after treatment is stopped. That means that the patient can be exposed to the drug, after the discontinuation of treatment.
There is a controversy over the duration of treatments and duration of drug holidays, and there are no evidence-based data to determine when and whether to resume treatment. Moreover, each bisphosphonate has a unique profile of bone affinity , and a difference in the speed of offset is highly expected within the bisphosphonates. Finally, the concept of drug holidays cannot be applied for drugs for which the effect resolves immediately after discontinuation.
Effect of Stopping Treatments
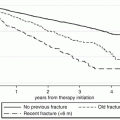
Bisphosphonates are the most popular treatment of osteoporosis and are widely prescribed. They are unique in their capacity to bind to bone matrix. Alendronate, ibandronate, risedronate, and zoledronate are different in the strength for binding to bone and in their potency for inhibiting farnesyl pyrophosphate synthase, their enzyme target. The high affinity of all bisphosphonates for bone is however a common property. When treatment is stopped, it is actually released from bone; because this release is function of the level of turnover, which is decreased by the presence of the bisphosphonate itself, this release can occur over months or years. This skeletal persistence can be associated with persistence of the clinical effect, for an unpredictable period of time. Whether or not the discontinuation is effective in avoiding side effects has been suggested in a large study, showing that the risk of atypical femur fractures associated with bisphosphonate use decreases immediately and significantly after discontinuation [1].
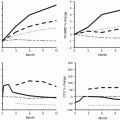
Long-term prospective extension trials are available for risedronate, alendronate, and zoledronic acid [2–4]. Hip and spine BMD decreases following discontinuation but remains above pretreatment levels after 1, 5, and 3 years of follow-up, respectively. Bone loss looks more rapid with risedronate than with the two other bisphosphonate, and this is in accordance to pharmacological properties.
The fracture risk after discontinuation of risedronate has been assessed after 3 years of treatment [2]. Initially 1628 patients were randomized to receive either placebo or risedronate 5 mg, and 759 entered a 1-year follow-up study; 599 completed the 1-year follow-up. The relative risk of morphometric vertebral fractures was still reduced by 46 % in the former risedronate group (incidence 6.5 %) compared to the former placebo group (incidence 11.6 %). In contrast, there was a −0.8 and −1.23 % decrease in lumbar spine and femoral neck BMD, in the previous risedronate-treated patients. Lumbar spine BMD, but not femoral neck BMD, was significantly higher than the former placebo group at the end of the extension year. For both lumbar spine and femoral neck, BMD values remained significantly higher than placebo at the end of year 4. Urinary NTX as a marker of bone resorption was available at the end of the follow-up in 89 patients of the former risedronate group: it increased from 30 to 51 nmol BCE/nmol creatinine. These data suggest that there is still a risk of fracture immediately after 3 years of treatment with risedronate but that this risk is lower than in untreated women. Surrogate markers, i.e., changes in BMD or biochemical markers over 1 year, cannot help to decide when to resume risedronate treatment, as their changes (decrease in BMD, increase in bone resorption) are not those expected in parallel to the persistent anti-fracture effect.
In FLEX study [3], patients previously treated by alendronate over 5 years were randomized to receive placebo or 5 additional years of alendronate. Those switched to placebo had a 1.5 % increase in lumbar spine BMD and a 3.38 % decrease in the hip BMD over 5 years. Biochemical markers were assessed in a subgroup of 87 patients, but data following immediately the discontinuation were not available: the first point of assessment of biochemical markers was at 3 years. A gradual rise in markers was measured: at 5 years, their value was 50–60 % higher than in patients who continued alendronate. Data on fractures were available in 1071 patients. There was no difference between placebo- and alendronate-treated patients for vertebral and non-vertebral fractures, except for clinical vertebral fractures with a lower incidence in the treated group than in the placebo group: 2.4 versus 5.3 %, respectively, over 5 years. This incidence is low and actually expected in this population, because of the selection at study entry, patients with a hip T score<−3.5 or with a decrease in hip BMD during the previous treatment period were excluded from this study. As a result, mean T score at baseline of the study were −1.3, −1.9, and −2.2 at the lumbar spine, total hip, and femoral neck, respectively. Only 30 % of the patients had osteoporosis based on a T score < −2.5 at the femoral neck; roughly 35 % of the population had a baseline vertebral fracture. Thus data were obtained in a population with a moderate risk of facture.
In the extension of HORIZON study [4], patients who switched to placebo after 3 years of zoledronic acid treatment had (after 3 years of follow-up) a BMD slightly lower than patients who continued treatment (with differences from 1.36 % to 2.06 % at the femoral neck and lumbar spine, respectively). There was no difference in markers of bone turnover at year 6 between patients who were continuously treated over 6 years or switched to placebo after 3 years of treatment. There was no difference in the incidence of vertebral and non-vertebral fractures between the two groups, except for morphometric vertebral fractures: 3 % versus 6.2 % over 3 years, respectively.
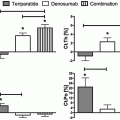
These results with bisphosphonates are different from those with other antiresorptive treatments , as their cessation results in immediate and large decrease in BMD. This is well known for estrogens, as hormone replacement therapy (HRT). After 2 years of treatment with conjugated estrogen 0.625 mg/day, women who switched to placebo experienced a 4.5 % and 2.4 % decrease at the spine and trochanter, respectively, over 1 year [5]. The post-intervention follow-up of the Women’s Health Initiative (WHI) trial showed that the risk of fractures was comparable among women in the previous HRT and placebo groups; this suggests a greater increase in the annualized risk of fractures in women after HRT therapy [6]. One year of discontinuation of raloxifene (after 5 years of administration) results in 2.4 % decrease in lumbar spine BMD [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree