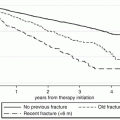
Fig. 19.1
Possible interactions between common causes of osteoporosis and fractures and cardiovascular events
In the industrialized world, the major causes of death are cardiovascular (including stroke) and cancer. Cancer will be described in a separate chapter below. A number of systematic searches were conducted using the PRISMA guidelines (http://www.prisma-statement.org/) for each of the ART categories (bisphosphonates, SERM, strontium ranelate, activated vitamin D) using the search term “mortality” or “cancer” and the drug in question. Extending the search to “survival” instead of mortality yielded more studies, but these were on cell lines. Studies on the use of these drugs to treat malignancy-related complications such as hypercalcemia or bone metastases [19] were excluded.
Bisphosphonates
A systematic search in PubMed using “bisphosphonates” and “mortality” per July 14, 2013 yielded 859 results.
Mortality in General in Users of Drugs against Osteoporosis
Bolland et al. performed a systematic review and meta-analysis of mortality in patients on drugs against osteoporosis [20]. The authors searched Medline and the Cochrane Central Register of Trials prior to September 2008, as well as the 2000–2008 American Society for Bone and Mineral Research conference abstracts.
Study Selection: Eligible studies were randomized placebo-controlled trials of approved doses of medications with proven efficacy in preventing both vertebral and non-vertebral fractures, in which the study duration was longer than 12 months and there were more than 10 deaths. Trials of estrogen and SERM were specifically excluded.
Data Extraction: Data were extracted from the text of the retrieved articles, published meta-analyses, or the Food and Drug Administration web site.
Data Synthesis: Eight eligible studies of four agents (risedronate, strontium ranelate, zoledronic acid, and denosumab) were included in the primary analysis. During two alendronate studies, the treatment dose changed, and those studies were only included in secondary analyses. In the primary analysis, treatment was associated with an 11 % reduction in mortality (relative risk, 0.89, 95 % confidence interval, 0.80–0.99, p = 0.036). In the secondary analysis, the results were similar (relative risk, 0.90, 95 % confidence interval, 0.81–1.0, p = 0.044). Mortality reduction was not related to age or incidence of hip or non-vertebral fracture , but was greatest in trials conducted in populations with higher mortality rates. However, this was the results of the combined drugs—for the individual bisphosphonates, no significant reduction was seen (alendronate, n = 2 studies; risedronate, n = 2 studies; and zoledronate, n = 2 studies). See also the sections for the individual bisphosphonates below [20].
Prior to this meta-analysis, several studies were identified that were not included. Cree et al. [21] performed an observational study. This study examined post-fracture osteoporosis drug treatment in hip fracture patients and the association of treatment with mortality and morbidity. Pre- and post-fracture demographic/health information was collected on a cohort of hip fracture patients aged 65+ years. Post-fracture administrative data on prescription drug use and health-care utilization was linked to the cohort data. Five classes of osteoporosis drugs were available during the study period: hormone replacement therapy, bisphosphonates, calcitonin, SERM, and vitamin D. Pre-fracture, 38 of 449 patients (8 %) were on osteoporosis medications. Post-fracture, 81 of 356 patients (23 %) were treated, and 63 of these patients were untreated prior to fracture. Both treated and untreated patients had similar rates of subsequent hip fracture (6 % and 4 %, respectively) and Colles fracture (2 %). Regardless of treatment status, patients were also equally likely to be hospitalized, both in the short-term (28 % in treated, 27 % in untreated) and in the long-term (43 % vs. 37 %). However, mortality was significantly lower in the treated group (long-term, OR = 0.34; 95 % CI: 0.17–0.70) [21]. These results, however, raise suspicion that “healthy drug user” effects may be in place. Those taking a drug may be healthier than those not adhering to a prescription. Steinbuch et al. [22] assessed mortality among users of risedronate enrolled in the North American Postmenopausal Osteoporosis Studies and reported no reduction in overall mortality (RR = 0.89, 95 % CI: 0.73–1.09)—see also below under risedronate.
Subsequent to the meta-analysis [20], a number of trials have been published. Sharma et al. [23] analyzed the risk of serious atrial fibrillation but also analyzed cardiovascular mortality in a meta-analysis and found no such excess (OR = 0.92, 95 % CI: 0.68–1.26). One study by Center et al. [24] using the Dubbo cohort (1223 women and 819 men aged 60+ years) reported that users of drugs against osteoporosis of whom there were 325 women (106 using bisphosphonates, 77 using hormone therapy, and 142 using calcium and vitamin D) and 37 men (15 on bisphosphonates, 22 on calcium and vitamin D) had a reduced risk of death compared to nonusers. In women, mortality rates were lower with bisphosphonates (0.8/100 person-years, 95 % CI: 0.4–1.4) and hormone therapy (1.2/100 person-years, 95 % CI: 0.7, 2.1) but not with calcium and vitamin D (3.2/100 person-years, 95 % CI: 2.5, 4.1) versus no treatment (3.5/100 person-years, 95 % CI: 3.1, 3.8) [24]. Accounting for age, fracture occurrence, comorbidities, quadriceps strength, and BMD, mortality risk remained lower for women on bisphosphonates [hazard ratio (HR) 0.3 (0.2, 0.6)] but not hormone therapy [HR 0.8 (0.4, 1.8)]. For the 429 women with fractures, mortality risk was still reduced in the bisphosphonate group [adjusted HR 0.3 (0.2, 0.7)], not accounted for by a reduction in subsequent fractures [24]. In men, lower mortality rates were observed with bisphosphonates but not calcium and vitamin D [BP 1.0/100 person-years (0.3, 3.9) and calcium and vitamin D 3.1/100 person-years (1.5, 6.6) versus no treatment 4.3/100 person-years (3.9, 4.8)] [24]. After adjustment, mortality was similar, although not significant [HR 0.5 (0.1, 2.0)] for men [24]. The main weakness of this study was the low number of treated especially among the men and the inability to adjust for adherence to the drugs. A study by Sambrook et al. [25] using frail elderly institutionalized subjects (n = 2005) showed a reduction in overall mortality among the oral bisphosphonate users (n = 78). Over 5 years of follow-up, 1596 participants (80 %) died. Use of bisphosphonates was associated with a 27 % reduction in risk of death compared to nonusers after adjusting for age, gender, type of institution, immobility, number of medications, weight, cognitive function, comorbidities, and hip fracture incidence during the follow-up period (hazard ratio 0.73, 95 % CI, 0.56–0.94, p = 0.02) [25]. Again the main limitation was the low number and the inability to adjust for adherence to the drugs and the type of bisphosphonate.
Mortality related to cancer will be described below in the section on cancer.
Prevention of Mortality following a Hip Fracture
Patients with a hip fracture have a high risk of subsequent death and a significant excess risk compared to the background population [16]. A randomized controlled trial in patients with a hip fracture showed a reduction in mortality with zoledronic acid (HR = 0.72, 95 % CI: 0.56–0.93) [26]. A subgroup analysis of patients from the HORIZON trial also using zoledronic acid in men with a recent hip fracture showed a similar trend toward a reduction in mortality [32/255 (13.1 %) vs. 51/261 (19.5 %), OR = 0.62, 95 % CI: 0.38–1.00, p = 0.05] but no reduction in cardiovascular events [27]. An observational study from Denmark showed that patients on bisphosphonates prior to a hip fracture had a reduced risk of death following the hip fracture compared to patients not on a bisphosphonate (OR, 0.68, 0.59–0.77) [28]. Also patients who began BP after the fracture (2.6 %) had significantly decreased mortality, both for patients who filled only one prescription (adjusted hazard ratio, HR 0.84, 0.73–0.95) and for patients who filled multiple prescriptions HR 0.73 (0.61–0.88) [28].
Myocardial Infarction and Cardiovascular Mortality
Some observational studies have suggested a reduced risk of myocardial infarction with the use of bisphosphonates in observational studies [29–31]. However, this was especially seen with high compliance [30]. The findings have to be confirmed from randomized controlled trials. Overall, no reduction in cardiovascular mortality was seen [22, 23].
Individual Bisphosphonates
Alendronate
A systematic search using “alendronate” and “mortality” produced 97 citations in PubMed. Two studies were included in the meta-analysis by Bolland et al. [20], the FIT trial by Black et al. [32] in postmenopausal women with prior spine fractures and the study by Cummings et al. [33] in postmenopausal women without spine fractures. None of the studies showed significant reductions in mortality, and the pooled estimate was 1.00, 95 % CI: 0.70–1.41 [20].
Clodronate
A systematic search using “clodronate” and “mortality” produced 105 citations in PubMed, but no studies fulfilled the inclusion criteria .
Etidronate
A systematic search using “etidronate” and “mortality” produced 64 citations, but no studies fulfilled the inclusion criteria.
Ibandronate
A systematic search using “ibandronate” and “mortality” produced 43 citations, but no studies fulfilled the inclusion criteria.
Pamidronate
A systematic search using “pamidronate” and “mortality” produced 101 citations, but no studies fulfilled the inclusion criteria.
Risedronate
A systematic search using “risedronate” and “mortality” produced 62 citations. Four studies reported on mortality, but these were partly overlapping.
The meta-analysis by Bolland et al. [20] included three RCTs, a study by Harris et al. [34] in postmenopausal women, a study by Reginster et al. [35] also including women, and a study by McClung et al. [36] including elderly women. The pooled estimate of the three trials was 0.88, 95 % CI: 0.70–1.10 for mortality with risedronate versus placebo. Besides the meta-analysis by Bolland et al. [20], Steinbuch et al. [22] studied 5303 patients exposed to either risedronate 2.5 or 5 mg daily and 2678 placebo-treated postmenopausal women included in the North American part of the risedronate registration studies. This study overlapped with the studies reported in the meta-analysis by Bolland et al. but also reported on causes of death. The study by Steinbuch et al. [22] did not find a reduction in overall (RR = 0.89, 95 % CI: 0.73–1.09), any cancer (0.89, 95 % CI: 0.59, 1.34), lung cancer (RR = 0.93, 95 % CI: 0.49–1.79), GI tract cancer (0.54, 95 % CI: 0.25–1.19), cardiovascular (0.54, 95 % CI: 0.25–1.19), coronary artery (1.15, 95 % CI: 0.72–1.84), stroke (0.50, 95 % CI: 0.29–0.88), or other cause mortality (0.97, 95 % CI: 0.66–1.42). Please also refer to the chapter on cancer below .
Zoledronate
A systematic search using “zoledronate” and “mortality” produced 224 citations and two original studies.
The meta-analysis by Bolland et al. [20] included both these studies, i.e., the study by Black et al. [37] and the study by Lyles et al. [26]. The combined estimate for mortality was 0.90, 95 % CI: 0.76–1.08. A number of subgroup analyses have been performed on the RCT of zoledronate following a hip fracture [26]. Eriksen et al. [38] performed a subgroup analyses by 2-week intervals of the time when the zoledronate infusion was administered after the hip fracture. For the times ≤2 weeks and 2–4 weeks after the hip fracture, no trend toward a reduction in mortality was seen [38]. For the time intervals 4–12 weeks, nonsignificant trends toward a reduction were seen, but the sample sizes were small, and from >12 weeks after the hip fracture, the risk reduction was statistically significant [38]. Upon pooling the results, patients treated >2 weeks following the hip fracture had a significant reduction in mortality, whereas those treated within two weeks did not. Patients dosed within 2 weeks were older and exhibited a higher baseline prevalence of hypertension, coronary artery disease, diabetes, atrial fibrillation, and stroke. These patients were also more likely to have come from an institutional setting before hospitalization for hip fracture and return to an institution after fracture compared with the subgroups dosed after the first 2 weeks [38]. Only 8 % of the reduction in mortality seemed attributable to the reduction of overall fracture risk after a hip fracture (tertiary prevention [39]) with zoledronic acid [40]. Other factors seemed to be reduced number of pneumonias and arrhythmias [40].
The excess risk of death following a hip fracture [16] is especially high within the first 6 months following the hip fracture owing to the frail nature of the patients sustaining hip fractures and the impact of the trauma. This may explain the absence of an effect of early administration of the drug in patients with a high general risk of death not particularly attributable to modifiable causes. Patients with hip fractures are very likely to sustain recurrent hip fractures [41], and this may explain why some of the reduction in mortality is linked to the prevention of recurrent hip fractures and thus death from these. A theoretical computation may be performed using the numbers from the trial by Lyles et al. [26]. In the placebo group, 33/1062 sustained a second hip fracture within the 3-year study period (and were thus alive at the time of fracture), while 141 died. In the zoledronate arm of the trial, 23/1065 sustained a second hip fracture, and 101 died. If it is assumed that the mortality following a hip fracture is double that of the background population [16], then the preventable deaths by avoiding a second hip fracture are 2.1 % (see also Table 19.1; the higher estimate by Colon-Emeric et al. [40] was due to the inclusion of other fractures besides hip fractures). Thus, even highly effective fracture preventing agents may only reduce overall mortality slightly if the reduction should come from hip fracture prevention alone.
Table 19.1
Potentially preventable deaths in percent of all deaths at different levels of absolute fracture risk and absolute risk of death, if the risk of death following a fracture is twice that of the background population and the fracture risk reduction is 50 %
Absolute fracture risk % | |||||
|---|---|---|---|---|---|
Absolute risk of death | 3 | 5 | 10 | 15 | 20 |
5 | 1.5 | 2.4 | 4.5 | 6.5 | 8.3 |
10 | 1.5 | 2.4 | 4.5 | 6.5 | 8.3 |
15 | 1.5 | 2.4 | 4.5 | 6.5 | 8.3 |
20 | 1.5 | 2.4 | 4.5 | 6.5 | 8.3 |
SERM
Raloxifene
A systematic search using the terms “raloxifene” and “mortality” produced 126 citations. Grady et al. [42] reported a pooled analysis of mortality data using two large clinical trials of raloxifene (60 mg/day) versus placebo, including the Multiple Outcomes of Raloxifene Evaluation/Continuing Outcomes Relevant to Evista studies (7705 postmenopausal osteoporotic women followed for 4 years and a subset of 4011 participants followed for an additional 4 years, 110 deaths) and the Raloxifene Use for the Heart trial (10,101 postmenopausal women with coronary disease or multiple risk factors for coronary disease followed for 5.6 years, 1149 deaths). Cause of death was assessed by blinded adjudicators. Cox proportional hazards regression models compared mortality by treatment assignment in a pooled analysis of trial data from the Multiple Outcomes of Raloxifene Evaluation/Continuing Outcomes Relevant to Evista and Raloxifene Use for the Heart trials. All-cause mortality was 10 % lower among women assigned to raloxifene 60 mg/day versus placebo (HR 0.90, 95 % CI: 0.80–1.00, p = 0.05). Lower overall mortality was primarily due to lower rates of non-cardiovascular deaths, especially a lower rate of non-cardiovascular, non-cancer deaths. This may be due to the fact that although raloxifene reduced breast cancer, this may be too rare an event for total mortality to be significantly decreased. However, it seems strange that a reduction was seen in non-cardiovascular, non-cancer deaths.
Bazedoxifene
One RCT in postmenopausal women reported no difference in mortality between bazedoxifene (17/1886 with 20 mg and 13/1872 with 40 mg), raloxifene (19/1849 with 60 mg), and placebo (11/1885) after 3 years [46].
Arzoxifene
One RCT in postmenopausal women reported no difference in mortality between arzoxifene (105/4676) and placebo (98/4678) [47].
Denosumab
A systematic search using “denosumab ” and “mortality” yielded 61 citations, but only one included mortality data and was included in the meta-analysis by Bolland et al. [20]. The study by Cummings et al. [48] in postmenopausal women reported a RR for mortality of 0.78, 0.57–1.06 with denosumab versus placebo.
Strontium Ranelate
A systematic search in PubMed using the terms “strontium ranelate” and “mortality” yielded 24 citations. Two studies were identified as included in the meta-analysis by Bolland et al. [20], the study by Meunier et al. [49] mostly including older women and the study by Reginster et al. [50] also including postmenopausal women. The combined estimate for mortality with strontium ranelate versus placebo was 0.94, 95 % CI: 0.77–1.15 [20].
Activated Vitamin D
A systematic search in PubMed using the terms “activated vitamin D” and “mortality” yielded 54 citations, but only provided papers on the survival in end-stage renal failure patients.
Theoretical Considerations
It is possible to simulate the proportion of all deaths in a population that in theory could be prevented by reducing the number of fractures. If assumptions are made in actual death rates, fracture rates, and the increased risk of death following a fracture, the theoretical proportion of all deaths potentially preventable through fracture prevention can be computed.
One may consider an example: say 20 % of a population fractures within a given time interval, i.e., 20 out of 100 suffer a fracture, whereas 80 do not. Suppose that 10 % of the no fracture cases dies (eight subjects) within that time frame and 20 % of the fracture cases dies (four cases as 20 % of 20 is 4). In total 8 + 4 = 12 or 12 % dies.
If fracture risk is reduced by 50 %, only 10 will fracture while 90 will not. With unchanged mortality rates, 10 % of 90 or 9 will die among the non-fracture cases, while 20 % of the 10 fracture cases or two subjects will die. In total, 9 + 2 = 11 will thus die although fracture risk is reduced by 50 %. The total mortality is thus reduced by 1/12 = 8.25 %. The relative risk of death thus is 11/12 = 0.92.
Tables 19.1 and 19.2 show simulations of the percent of all deaths that could be avoided by preventing fractures at various levels of absolute fracture risk and risk of death. Even at high fracture risk and risk of death, only a minor fraction of total mortality could be prevented. As can be seen, the proportion of deaths prevented is independent of the absolute risk of death (however, the absolute number of deaths potentially preventable of cause increases). A mortality reduction may thus only be achieved with long-term treatment, and here adherence with ART over prolonged time intervals may be an issue [51].
Absolute fracture risk % | |||||
|---|---|---|---|---|---|
Absolute risk of death | 3 | 5 | 10 | 15 | 20 |
5 | 2.0 | 3.3 | 6.4 | 9.1 | 11.7 |
10 | 2.0 | 3.3 | 6.4 | 9.1 | 11.7 |
15 | 2.0 | 3.3 | 6.4 | 9.1 | 11.7 |
20 | 2.0 | 3.3 | 6.4 | 9.1 | 11.7 |
In the studies that allowed evaluation of both mortality risk and risk of any fracture [26, 32, 33, 37, 47], the absolute risk of any fracture was 13 % in the placebo group, and the risk of death was 3 %, i.e., in theory 5.8 % of the deaths were preventable with a 50 % reduction in fracture risk and a doubling of mortality risk following a fracture. This would equal an RR of death of 0.94. If the scenario was altered to a 30 % reduction in fractures, 3.5 % of deaths could be prevented or an RR of 0.96. This is actually in the range observed by Bolland et al. for most drug categories [20]. Only in the study by Lyles et al. [26] was a reduction in the risk of death seen, but this was also a study with a very high mortality rate (13 % in the placebo group) and a high fracture rate (13 % in the placebo group). The other studies may have high fracture rates, but had low mortality rates, i.e., there were few deaths to prevent. In the combined MORE/CORE study on raloxifene, a reduction in mortality was reported despite an absence in a reduction in overall fracture rates, but this may have been related to effects on other factors including cancer deaths (see below) [42]. Bolland et al. [20] suggested an 11 % reduction in mortality, the estimate being independent of hip and non-vertebral fracture rates, but higher at higher mortality rates [52]. This may be due to the fact that hip and vertebral fracture rates in general were low and the number of preventable fracture-related deaths thus low. Prevention of non-fracture-related deaths may be a possibility, but besides the raloxifene-related breast cancer deaths (which were not included in the meta-analysis by Bolland et al. [20]), none of the studies have pinpointed specific causes of death that were preventable by ART.
Conclusions
Overall ART may not reduce mortality, as only a minor part of deaths can be attributed to fractures. Mortality reductions from fracture prevention may only be achieved in populations at high risk of both fractures and death (i.e., typically the elderly and especially subjects with a prior hip fracture). Unless ART have effects on other causes of death, mortality reductions may thus not be seen. Studies to address the effects of dose and duration as well as adherence to ART and effects of ART on other causes of mortality are warranted. Almost no studies have included men as the majority has dealt with postmenopausal women.
Cancer Incidence
A number of the ART may also be used in oncology to treat bone-related events such as hypercalcemia and skeletal metastases (the bisphosphonates and denosumab) and to antagonize estrogen receptor-positive breast cancer (raloxifene and other SERMs such as tamoxifen). However, this is not the focus of this review, which will focus on the effects on cancer incidence by the drugs per se.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree