57.1
Bone health management in prostate cancer
57.1.1
Introduction
Prostate cancer (PCa) is a common cancer in men, with a lifetime risk of approximately 11.6% and is the second most common cancer in men worldwide. An estimated 175,000 new cases were diagnosed in 2019 or almost 10% of new cancer cases, with close to 31,000 deaths. Approximately 77.2% are diagnosed at an early stage, with a 5-year survival for localized PCa of 98%. The median age at diagnosis is 66 years, and it is more likely to occur in African-American men and in those with a family history of PCa . There are currently over 3.5 million survivors of PCa in the United States, and it is estimated that by the year 2030 the number of PCa survivors will be more than 5 million . In addition, many men with PCa will develop skeletal metastases during the course of their disease .
Since testosterone is an important stimulator of PCa cell proliferation until castrate resistance occurs, androgen deprivation therapy (ADT) is used in various stages of the disease, and 33%–70% of men will receive ADT in the course of their disease . This treatment will render men hypogonadal and thus be accompanied by significant bone loss and consequent fractures. There are also other important side effects of ADT, including sarcopenia, vasomotor flushing, acceleration of the frailty syndrome, and an increase in the risk of diabetes mellitus and thromboembolic events . However, the focus of this review will be on the preservation and management of bone health and fracture prevention in men with localized PCa. This is a growing public health concern as fractures lead to significant morbidity and mortality, and the burden of disease will increase with the aging of our population.
57.1.2
Normal bone physiology
Continual ongoing remodeling is an important characteristic of normal bone physiology and is dependent upon a balance between osteoclast and osteoblast activity (as discussed elsewhere). Osteoclasts are principally responsible for bone resorption. Mature osteoclasts are a product of monocyte/macrophage differentiation near the bone surface. They bind to the bone surface and create a resorption pit into which they secrete protons and lytic enzymes such as cathepsin K and tartrate-resistant acid phosphatase. This resultant resorption of bone liberates calcium, phosphate, collagen fragments, and a variety of growth factors such as transforming growth factor and insulin-like growth factors . Osteoblasts will subsequently lay down osteoid that will ultimately be mineralized; thus osteoblasts are responsible for the formation of new bone matrix. In young healthy bone the activities of these two cell types are coupled such that bone mass is largely static. Perturbations in these coupled processes occur with menopause in women and with aging in both genders. When superimposed on these universal phenomena, chemotherapies or hormonal manipulation lead to further imbalances in bone remodeling, loss of bone, and the clinical consequence of fracture.
The regulation of bone remodeling is complex. Receptor activator of nuclear factor kappa B (RANK) is the signaling pathway that is thought to be most central to the regulation of osteoclast differentiation and function. One source of RANK ligand (RANKL) is the osteoblast. RANKL induces osteoclast differentiation by binding to RANK on osteoclast precursors. RANKL can also lead to osteoclast activation and survival by binding to RANK on mature osteoclasts. Osteoprotegerin (OPG), also a product of the osteoblast, downregulates RANKL signaling by serving as a decoy receptor for RANKL. Thus it serves to protect bone from excess resorption. The balance of these two cytokines, RANKL and OPG, plays a major regulatory role in bone mass and strength . Further details of this complexed cell-to-cell interactions are discussed elsewhere in depth in several chapters in this volume.
57.1.3
Androgen deprivation therapy and hypogonadism
As localized disease accounts for the majority of cases of PCa, options include “watchful waiting” or surveillance, radiation treatment with or without ADT or radical prostatectomy. Localized disease can be characterized by low, intermediate, or high risk and is determined by Gleason score, prostate-specific antigen as well as clinical stage. Decision-making regarding treatment is based on risk stratification, other comorbidities, and life expectancy.
Marked hypogonadism is the intended therapeutic effect of ADT for men with PCa, as testosterone is a growth factor for PCa cells. The current prevalence of ADT use is high . It is supported by level 1 evidence of benefit for men with PCa in several specific clinical situations. First, it is used as neoadjuvant, concomitant, or adjuvant therapy in combination with external beam radiation for intermediate- and high-risk localized disease. Men with intermediate-risk tumors generally receive 4–6 months of ADT , while men with high-risk tumors generally receive 2–3 years of ADT due to demonstration of overall survival benefit . Second, adjuvant ADT can be given to men who have undergone prostatectomy for node-positive disease . Third, ADT is the foundation of systemic therapy for advanced PCa and may also commonly be given for the treatment of biochemical recurrence after primary therapy, a setting in which benefit is less certain.
ADT can be accomplished in a number of ways. The most common method is medical castration with the use of a gonadotropin-releasing hormone (GnRH) agonist to eliminate testicular production of androgens by antagonizing gonadotropin release at the level of the hypothalamus. This leads to an initial transient stimulation of luteinizing hormone from the pituitary but ultimately to downregulation of pituitary receptors and castrate levels of testosterone within 2–3 weeks . ADT can also be accomplished by degarelix, a GnRH antagonist that produces similar levels of longitudinal androgen suppression without a transient initial rise in androgen production . Androgen receptor antagonists such as bicalutamide and nilutamide) block the action of testosterone and adrenal androgens on the androgen receptor and are often combined with GnRH-agonist treatment. These agents are used before and during the first weeks of ADT with GnRH agonists to block the testosterone surge and to establish what is termed total androgen blockade . However, they are not considered standard forms of ADT if used without concomitant elimination of gonadal androgen synthesis. Finally, androgen suppression can be accomplished surgically with bilateral orchiectomies, although not commonly performed in the current era.
Medical or surgical elimination of gonadal androgen production brings about significant changes in the hormonal environment of men with PCa. In addition to the decline in testosterone to castrate levels ≤50 ng/mL , ADT is also associated with low estrogen levels, as estrogens are derived from aromatization of androgens. Both estradiol and testosterone levels have been independently correlated with bone mineral density (BMD) , bone turnover , and fracture risk among men in the general population. In one study the mean estradiol level among men receiving ongoing ADT was 12 ng/dL , a level that corresponds to the lowest quartile of men in the general population and is associated with elevated fracture risk .
57.1.4
Effects of androgen deprivation therapy on BMD, fracture, and muscle mass
Osteoporosis-related fractures cause considerable morbidity and mortality among men in the general population. Twenty percent of those who meet criteria for OP or osteopenia are men, and 30%–40% of fractures due to osteoporosis occur in men . Worldwide, the age-standardized incidence of hip fracture in men is approximately half of that in women . Hip fractures carry a high risk of mortality in men than women. After hip fracture, men in a large prospective Australian cohort had a higher mortality rate than women when compared with age- and sex-specific expected survival rates in the overall population .
It has been well established in a number of prospective studies that ADT leads to accelerated bone loss and increased bone turnover as seen in Fig. 57.1 . The use of GnRH-agonists among men with nonmetastatic PCa is associated with an annual loss of 0.6%–4.6% in BMD, with the greatest rate of bone loss occurring during the first year of therapy at both the spine and hip sites . According to the US data, a fracture in a man with PCa more than doubles mortality in men on ADT, with or without metastatic disease, after adjustment for covariates such as age, tumor grade and stage at diagnosis, race, and other comorbidities .
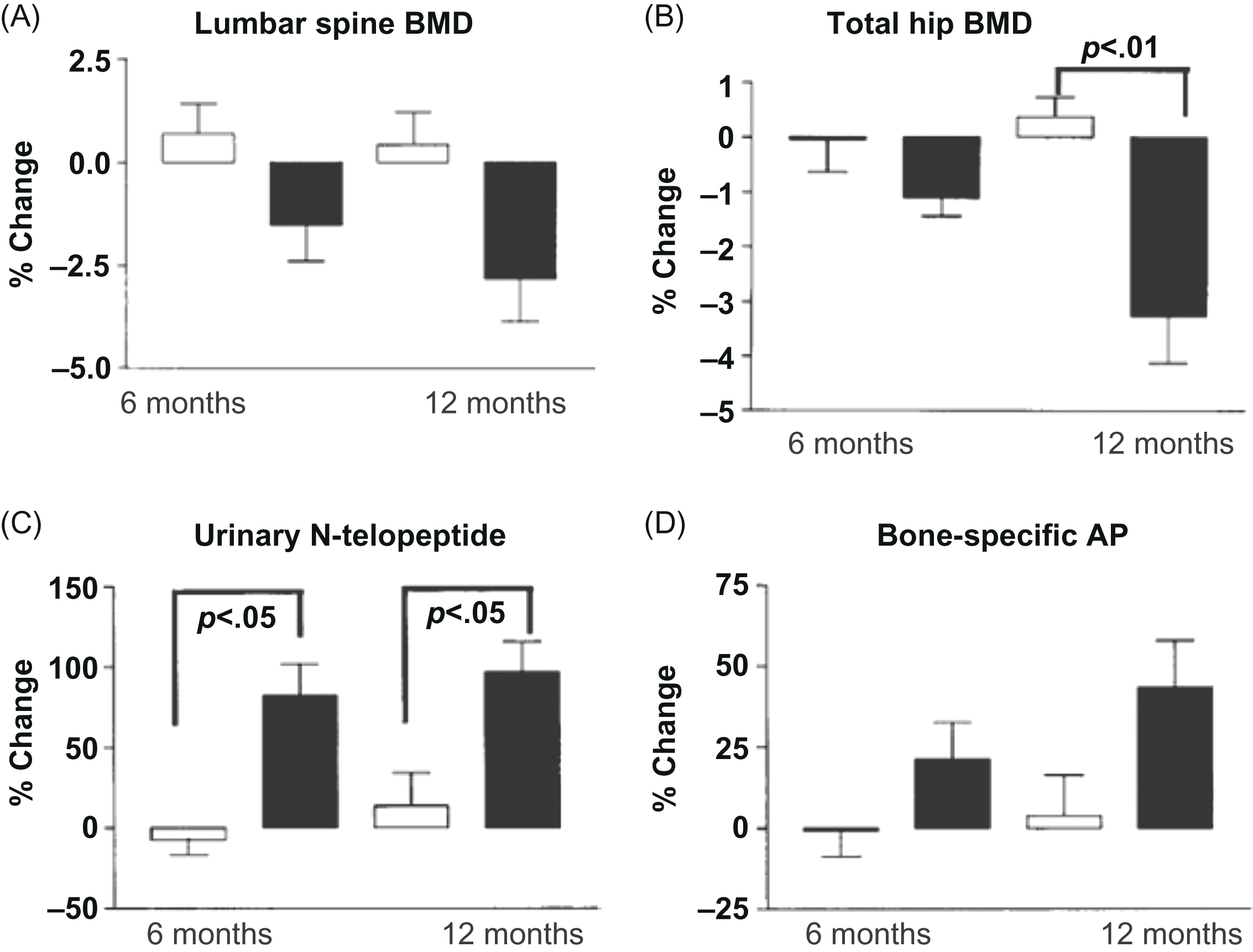
Given that low BMD is associated with elevated fracture risk, two notable population-based studies examined the incidence of osteoporotic fracture among men receiving ADT. Shahinian et al. studied records of over 50,000 men with PCa within the Surveillance, Epidemiology, and End Results (SEER) database. They found that among men living at least 5 years after the diagnosis of PCa, 19.4% of those who received ADT had a fracture as compared with 12.6% of those who had not received ADT ( P <.001) . The investigators commented that available data from other studies showed older age, prior fracture, dementia, and lower BMD at initiation are associated with both ADT and fracture . However, in the Shahinian study, the association between fracture risk and number of treatment doses remained even after adjustment for known confounders . Smith et al. carried out a claims-based cohort study using a sample of Medicare beneficiaries with nonmetastatic PCa. They found that GnRH-agonist treatment was associated with an elevated risk of any clinical fracture [7.88 per 100 person-years at risk with treatment compared with 6.51 per 100 person-years in matched controls; relative risk 1.21, 95% confidence interval (CI) 1.14–1.29; P <.001], vertebral fractures (relative risk 1.45, 95% CI 1.19–1.75; P <.001), and hip/femur fractures (relative risk 1.30, 95% CI 1.10–1.53; P =.002) during 7 years of follow-up . In another cohort analysis of 80,844 patients using SEER data, investigators found that fracture rates increased with increasing cumulative GnRH dose but, importantly, risk of fracture declined with increased number of months off ADT . Finally, in a recent study of close to 180,000 men with a mean age of 80 years from a large Swedish registry, investigators compared 20,000 men with PCa with and without ADT. Compared to men without PCa, those with PCa and ADT had an increased risk of any fracture [HR 95% CI 1.40 (1.28–1.53)], hip fracture [1.38 (1.20–1.58)], and major osteoporotic fracture [1.44 (1.28–1.61)], while patients with PCa not receiving ADT had a similar fracture risk to men in the general population. They also found no significant relationships between treatment with ADT and incident falls (not leading to fracture), lending evidence that the relationship between ADT and fracture is primarily bone related. The most important risk factors for incident fracture were advanced age, low BMI, previous fracture, and/or previous falls; however, other usual risk factors seen in risk assessment models such as the Fracture Risk Assessment model (FRAX) were not significant in their statistical analysis. The authors concluded men treated with ADT are a particularly frail group at high fracture risk who should be closely monitored .
Another consequence of the hypogonadism due to ADT is loss of muscle mass and function, termed sarcopenia. As described in men initiating ADT, decreased lean mass and increased body fat mass occur as early as 3–6 months . In a systematic review of longitudinal studies, a mean increase of body fat of 7.7% and a reduction in lean body mass of 3% was described, with longer duration of ADT use associated with more extensive changes . Sarcopenia has been associated with fractures due to falls, as well as decreased BMD and changes in bone geometry .
57.1.5
Radiation therapy and hip fracture
Many men with PCa undergo radiation therapy (RT) as part of their treatment. Radiation therapy can affect mature bone by direct damage to osteoblasts and indirectly cause vascular injury with weakening of bone and potential for fracture . Investigators analyzed data in over 45,000 men obtained from the SEER cancer registry of men with nonmetastatic PCa within 6 months of diagnosis. Men over 66 years of age who received external beam RT alone had a 10-year risk of hip fracture of 8%, and with the addition of ADT to RT (mean 11 months) the risk increased to 9%. Hip fracture risk with ADT alone was 16% (mean duration of ADT of 19 months) and men treated with radical prostatectomy alone had the lowest risk, 2.6%; however, this group was the youngest and had the fewest comorbidities .
57.1.6
Osteoporotic fractures versus disease-related fractures
It is important to make the distinction between fracture risk due to treatment-related osteoporosis and fracture risk due to PCa that has metastasized to bone. The former is the focus of this chapter. However, skeletal morbidity due to PCa bone metastases is a major clinical problem, as metastasis to bone is extremely common in men with advanced PCa with a prevalence of 80%–90% in typical clinical trials . A number of trials have examined therapies that affect osteoclast inhibition for the prevention of pathologic fractures, spinal cord compression, need for surgery to bone, or need for RT, the composite of which is termed skeletal-related events. These trials have generally featured monthly therapy with potent agents at a much greater cumulative drug exposure than is used for the treatment of osteoporosis. The two drugs that are indicated for this clinical setting are zoledronic acid and denosumab .
57.1.7
Risk assessment in men with prostate cancer
As many men receive long-term ADT, appropriate management of fracture risk in this population is an important survivorship issue. The National Osteoporosis Foundation (NOF) and the American College of Preventive Medicine recommend that all men aged 70 or older be screened for osteoporosis using DXA . DXA usually includes spine and hip; however, forearm is an option when either of these sites is not evaluable (e.g., due to spine arthritis and/or hip replacements). In the United States, over half of the PCa diagnoses are in men aged 65 and over, with a median age of death from the disease of 80 years . Thus bone health is an important and often overlooked issue in this population of older men.
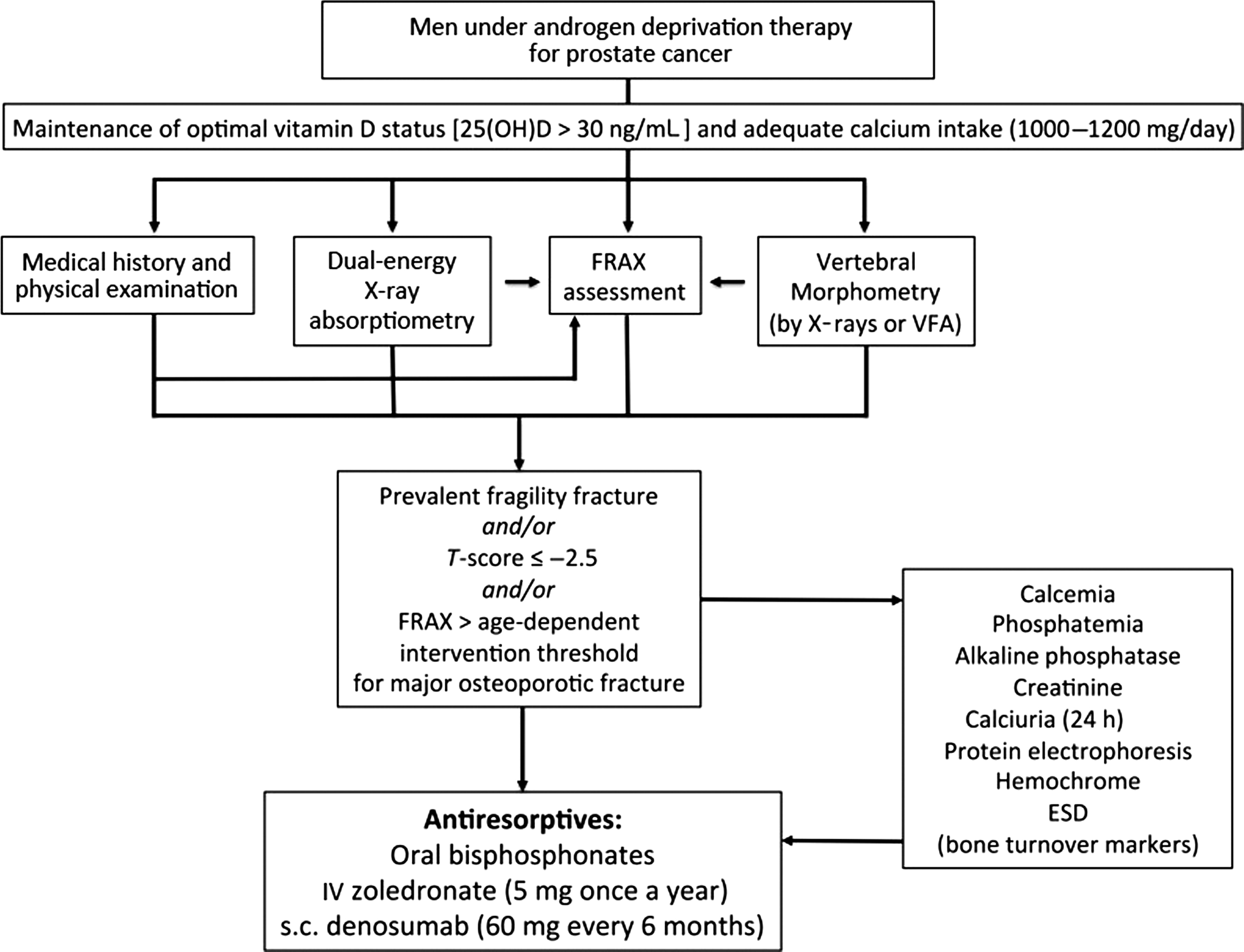
Although risk assessment tools have not been validated in patients with cancer, the National Comprehensive Cancer Network (NCCN) guidelines for the management of PCa advocate the use of the World Health Organization FRAX tool to determine fracture risk among men receiving ADT for PCa. The FRAX model is available online ( http://www.shef.ac.uk/FRAX ) and can estimate 10-year fracture risk with or without the use of measured femoral neck BMD. Clinical inputs include gender, age, calculated BMI, history of fracture, parental history of hip fracture, smoking status, use of glucocorticoids, daily consumption of at least 3 U of alcohol, rheumatoid arthritis, and other causes of secondary osteoporosis (e.g., untreated hypogonadism or thyroid disorders, inflammatory bowel disease, type 1 diabetes, and prolonged immobility) . Identification of men who will benefit from antiosteoporosis therapy requires information, including a baseline BMD that can be put into the FRAX algorithm, and is recommended by NCCN and American Society for Clinical Oncology (ASCO) . Given that the majority of fragility fractures in men occur in the setting of a nonosteoporotic BMD (i.e., BMD T -score ≥−2.5) , these additional risk factors are important to consider. ASCO guidelines recommend that patients with nonmetastatic cancer and at least one risk factor for osteoporosis be screened at treatment initiation with BMD testing via central dual-energy X-ray absorptiometry (DXA) .
Recommendations for repeat DXA testing are variable. Guidelines from the International Osteoporosis Foundation suggest that high-risk patients treated with antiresorptive or those at low risk (e.g., normal BMD at initiation of ADT) have repeat BMD every 18–24 months. Further, they suggest that men with osteopenia who are not treated with anti-resorptive medication at ADT initiation should be monitored on an annual basis to determine if rapid bone loss requiring treatment has occurred . Fractures occurring on ADT may change decision-making, and indeed, clinical judgment is essential for the best outcomes in individual patients.
The NOF guidelines recommend the use of drug therapy to reduce fracture risk if a 10-year risk exceeds either of two thresholds: >20% risk of major osteoporotic fracture or >3% risk of hip fracture . When the FRAX algorithm is applied to men receiving ADT for PCa, a substantial percentage of men are identified as candidates for drug therapy. When using FRAX in men on ADT, secondary osteoporosis may be given a “yes” designation. Several studies have determined that when FRAX (without BMD data) is compared with conventional T -score alone, more men are deemed to require treatment, and older age is the most important risk factor . An overall algorithm for risk assessment and management of men with nonmetastatic PCa on ADT is shown in Fig. 57.2 .
57.2
Nonpharmacologic therapies
57.2.1
Calcium, vitamin D, and exercise
Dietary guidelines for calcium and vitamin D have been set by the Institute of Medicine (IOM), which recommends that men aged 51–70 years have 1000 mg calcium and 600 IU vitamin D. For men aged >70 years the recommendation is 1200 mg calcium and 800 IU vitamin D Calcium intake through dietary sources is optimal, but supplements can be used to achieve adequate daily intake. Specific guidelines for the optimal doses of calcium and vitamin D to prevent bone loss in this population have not been established . In an analysis of 12 clinical trials, commonly recommended doses of 500–1000 mg calcium and 200–500 IU vitamin D per day in men on ADT were insufficient to prevent bone loss . However, a recent study of men (mean age 67 years) with PCa on ADT showed that the group on high-dose vitamin D (50,000 units weekly plus vitamin D 600 IU/day) and who achieved a serum level of >32 ng/mL had a significantly improved “phase angle” parameter, a potential bioelectrical indicator of sarcopenia related to muscle mass and strength in older individuals. This was in comparison to a placebo group on vitamin D 600 IU/day with levels of vitamin D that increased by 4.3 ng/mL from baseline (levels not shown). . It has been shown, in a study of women treated with aromatase inhibitors for breast cancer in which supplements of 800 IU/day of vitamin D was given, that bone loss over the first year of AI therapy was reduced in those subjects attaining a level of ≥40 ng/mL compared with those of <30 ng/mL . The question of whether vitamin D has a protective or preventive role in PCa has been evaluated. A nested case–control cancer prevention study found a lower risk of PCa in men with moderate levels of vitamin D (approximately 45–70 nmol/L) compared with men with lower or higher values. This risk was also stronger for high-grade disease .
Although recommendations for exercise usually include balance training, stretching and flexibility, endurance exercise, progressive strength, and resistance training to lower fall risk, data on the effect of exercise on BMD are limited. Several metaanalyses and trials investigating aerobic and/or resistance exercise in men on ADT have not been shown to improve BMD in this population . In one study of recreational soccer training in elderly European men with advanced or locally advanced PCa treated with ADT, BMD was maintained compared with a control group for up to 5 years of follow-up, although this was in a small number of men that continued to participate in soccer one-to-two times per week . Overall recommendations for exercise by the NOF in adults include weight-bearing and muscle-strengthening exercise to improve agility, strength, posture, and balance to decrease fall risk ; however, these different exercise modalities may require modification in individuals with medical comorbidities and/or physical limitations.
In addition to the above mentioned considerations, counseling on smoking cessation and safe alcohol consumption is the foundation of a healthy lifestyle that should be addressed in this population. In addition, attempts at reducing the dose or eliminating medications that have adverse effects on the skeleton should be considered.
57.2.2
Pharmacologic therapies
As per earlier discussion, ADT in men with PCa has been documented to lead to accelerated bone loss and increased risk of fractures; therefore medical treatment is indicated in those men at high risk. Medications approved for treatment in men with PCa include both oral and intravenous (IV) bisphosphonates (BPs) as well as denosumab.
The BPs represent the first class of medication to be shown to improve BMD in men receiving ADT and remain as commonly prescribed agents in this population. Bisphosphonates that have been shown to improve BMD include alendronate, risedronate, pamidronate, and zoledronic acid (ZOL). Oral and intravenous BPs have been shown to prevent bone loss and to increase BMD at the l -spine and hip in men on ADT; however, data on fracture efficacy are lacking in most studies since they were not powered for that end point . Interestingly, ZOL was initially evaluated and approved for hypercalcemia of malignancy, and subsequent trials assessed the effect on BMD in various dosing schedules. One trial evaluated a single annual IV ZOL infusion and showed that at 12 months L-spine BMD decreased by 3.1%±1.0% in men given placebo and increased by 4.0%±1.0% in men who received ZOL ( P <.001); BMD of the total hip decreased by 1.9%±0.7% in men in the placebo group and increased by 0.7%±0.5% in those on ZOL, ( P =.004) . Overall the ZOL trials for a duration of 1–3 years have shown that regardless of ADT regimen, dosing interval, or baseline BMD, gains in bone mass are demonstrated, although vertebral fracture prevention has not been shown even in the longest trial. Thus the optimal regimen for ZOL is uncertain. A listing of BP studies are shown in Table 57.1 .
| Study | Treatment | Patient population | Duration | Drug regimen | Prevention of bone loss | Fracture prevention |
|---|---|---|---|---|---|---|
| Smith et al. | Pamidronate | N =47, locally advanced, lymph node-positive or recurrent PCa | 48 weeks | IV 60 mg Q 3 months | Yes | No |
| Greenspan et al. | Alendronate | N =112, on ADT or antiandrogen or combination Rx | 12–24 months | P.O. 70 mg/week | Yes | No |
| Klotz et al. | Alendronate | N =191, initiation of ADT | 12 months | P.O. 70 mg/week | Yes | No |
| Choo et al. | Risedronate | N =104, local advanced PCa | 24 months | P.O. 35 mg/week | Yes | No |
| Taxel et al. | Risedronate | N =40, nonmetastatic PCa, initiation of ADT | 6 months | P.O. 35 mg/week | Yes | No |
| Smith et al. | Zoledronic acid | N =106 | 12 months | 4 mg IV Q 3 months | Yes | No |
| Denham et al. | Zoledronic acid | N =1071, locally advanced PCa, initiation of PCa | 36 months | 4 mg IV Q 3 months | Yes | No |
| Michaelson et al. | Zoledronic acid | N =40, nonmetastatic PCa | 12 months | 4 mg IV/year | Yes | No |
| Smith et al. | Denosumab | N =1468, on ADT | 36 months | 60 mg Q 6 months | Yes | Yes (vertebral) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








