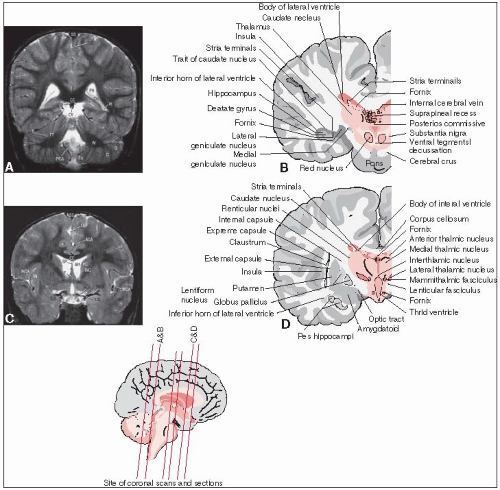
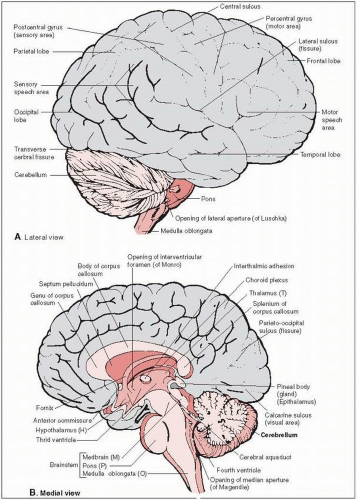
The tentorium cerebelli, which consists of dense fibrous tissue, separates the supratentorial and infratentorial compartments (see anatomical drawings in Figs. 11-1 and 11-2).
In the supratentorial cerebrum, primary motor and somatosensory areas on opposite sides of the central sulcus (motor in precentral gyrus; somatosensory in postcentral gyrus) control the body from the knees to the feet in the medial cortex, and the trunk, arms, and head in the lateral cortex (homunculus).
The motor-speech area of Broca is located posteriorly in the dominant frontal lobe just superior to the lateral sulcus; damage causes expressive aphasia. Damage to the dominant temporal lobe at the posterior end of the superior temporal gyrus immediately inferior to the lateral sulcus (Wernicke’s area) results in receptive aphasia.
Most of the primary visual cortex is represented on the medial and inferior surface at the occipital pole.
The telencephalon consists of the cerebral cortex, basal ganglia, and olfactory bulb.
The diencephalon includes the thalamus, hypothalamus, posterior pituitary, and pineal region.
The mesencephalon rides on the upper part of the clivus at the tentorial notch; its interior is partially occupied by cranial nerve nuclei (for the oculomotor, trochlear, and proprioceptive portion of trigeminal nerves).
The mesencephalic dorsal plate (tectum) houses the superior and inferior colliculi, which regulate eye movements/visual processing and auditory processing, respectively; the trochlear nerve is the only cranial nerve that exits from this dorsal location.
The mesencephalic ventral region (cerebral peduncles) includes the midbrain tegmentum, substantia nigra (extrapyramidal motor function), and crus cerebri (houses longitudinal tracts).
The pons (part of the metencephalon along with the cerebellum) relays information between the two cerebellar hemispheres, carries the major pathways from the mesencephalon down to the medulla oblongata, and houses the major motor and tactile sensory nuclei for the trigeminal nerve, which emerges from its lateral surface.
The border between the pons and the medulla oblongata is noteworthy for the emergence of the abducens, facial, and vestibulocochlear (acoustic) cranial nerves.
The cerebellum develops laterally and posteriorly from the pons region and differentiates into the median vermis cerebelli and bilateral hemispheres. Anteriorly, the cerebellum faces the dorsal aspects of the pons and the medulla oblongata (in the form of the floor of the fourth ventricle).
The medulla oblongata (portion of myelencephalon) forms the link between the pons, spinal cord, and cerebellum; it houses cranial nerve nuclei (glossopharyngeal, vagal, accessory, hypoglossal) and is implicated in the control of important autonomic functions.
The ventricular system is lined with ependyma and produces cerebrospinal fluid (CSF) in the roofs of the fourth and third ventricles, the medial walls of the central body, and the inferior horns of the lateral ventricles.
The foramina of Monro transmit CSF between the third and lateral ventricles at the superolateral corners of the third ventricle.
The aqueduct of Sylvius in the midbrain (connecting the third and fourth ventricles) is the narrowest canal of the intracranial nervous system and is also the most common location of obstruction of flow by compression, which causes noncommunicating (obstructive) hydrocephalus.
CSF escapes the ventricular system into the subarachnoid space through the median foramen of Magendie and the two lateral foramina of Luschka (which are located in the roof and lateral corners of the fourth ventricle at the level of the medulla oblongata).
The subarachnoid space widens into several cisterns; the largest are the cisterna magna (posterior to medulla oblongata just at foramen magnum), the cistern of the lateral sulcus bilaterally at the base of the brain, and the ambient cistern posterior to the midbrain.
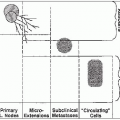
Some primary brain tumors have well-defined borders (e.g., pilocytic astrocytomas), whereas many such as high-grade gliomas tend to spread invasively in the brain.
Intracranial primary neoplasms do not metastasize through the lymphatics.
Extracranial true metastases from primary brain tumors are rare, but sometimes can occur with high-grade medulloblastoma, germinoma, hemangiopericytoma, sarcoma, and high-grade astrocytoma. These hematogenous metastases often appear in the lung; medulloblastoma has an affinity for bone, bone marrow, and lymph nodes.
Peritoneal metastases infrequently occur in patients receiving ventriculoperitoneal shunts to relieve obstructive hydrocephalus from tumors.
Some high-grade neoplasms in the brain and meninges metastasize by “seeding” into the subarachnoid and ventricular spaces and in the spinal canal, particularly in patients with recurrent tumors. Tumors with a propensity for such CSF spread include medulloblastoma, ependymoblastoma, pineoblastoma, and central nervous system (CNS) lymphomas.
Presenting signs and symptoms depend on tumor location, associated expansion, and surrounding edema.
Local tumor growth, edema, or both may cause focal neurologic dysfunction, increased intracranial pressure, and/or hydrocephalus. The neurologic effects of an intracranial tumor may be somewhat predicted by its location. With significant cerebral edema or hydrocephalus, signs and symptoms of increased intracranial pressure (e.g., nausea/vomiting, headache, papilledema) and generalized cerebral dysfunction may predominate.
Increased intracranial pressure or local pressure on sensitive intracranial structures (dura and vessels) may cause headaches, which may be worse in the morning. Associated findings include focal neurologic deficits and papilledema.
Long-standing increases in intracranial pressure may lead to optic atrophy and blindness.
Seizures are common and may be partial (simple, complex, secondarily generalized) or generalized (tonic-clonic, absence); the highest incidence is with low-grade neoplasms.
Lumbar back pain or bowel or bladder dysfunction may suggest CSF metastasis in the lumbar cistern.
The initial workup of patients with brain tumors must include a complete history and a general physical examination.
Complete neurologic examination includes assessment of mental condition, cranial nerves, coordination/cerebellar function, sensation, power, and reflexes.
Ophthalmoscopy checks for papilledema as a sign of increased intracranial pressure.
TABLE 11-1 Differential Diagnosis of Space-Occupying Lesions on CT or MRI | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||
The most commonly useful magnetic resonance imaging (MRI) studies are T1-weighted images (with and without gadolinium contrast), T2-weighted images, and fluid attenuated inversion recovery (FLAIR) images. T1 images better demonstrate anatomy and areas of contrast enhancement (CE); T2 and FLAIR images are more sensitive for detecting edema and tumor hyperintensity. Computed tomography (CT) scans with contrast material also are useful. See Table 11-1.
Staging of the neuraxis is essential for neoplasms at high risk of spread to the CSF. Neuraxis imaging is usually achieved with gadolinium-enhanced MRI of the spine. Ideally, neuraxis imaging should be done preoperatively to avoid surgical artifacts and false-positive scans. Spinal imaging is usually combined with CSF cytology for complete neuraxis staging.
Biopsy of a CNS tumor is generally recommended, but often tissue diagnosis is achieved through frank resection. Selected patients with imaging and symptoms consistent with lowgrade glioma may be followed closely without biopsy.
CSF cytology is essential for staging tumors with a propensity for CSF spread (e.g., germ cell tumor, primitive neuroectodermal tumor [PNET], medulloblastoma, CNS lymphoma). CSF sampling in the immediate postoperative period may lead to false-positive studies and is best done either preoperatively or at least 2 to 3 weeks postoperatively.
A recursive partitioning analysis by the Radiation Therapy Oncology Group (RTOG) showed that patients with brain gliomas stratified according to age, Karnofsky performance status, histology, mental status, extent of surgery, time between symptoms and treatment onset, neurologic function, and irradiation dose could be classified into six groups with regard to prognosis and response to treatment (17, 94). Chang et al. (11) proposed a staging system for medulloblastoma, although modern protocols classify patients as either standard-risk (age more than 3 years, gross total resection or subtotal resection with less than 1.5 cm3 residual, and no metastases) or high-risk (do not meet standard-risk criteria).
Primary intracranial tumors arise from the brain, cranial nerves, meninges, pituitary, and vessels and derive from the ectoderm (brain) and mesoderm (vessels, meninges, blood components).
The 1979 World Health Organization (WHO) classification of primary CNS tumors lists distinct pathologic subtypes of CNS tumors in broad histologic categories (6).
Histopathologic grade of tumor is important because benign lesions generally have a better prognosis and may be cured or controlled with surgery or irradiation.
Age, tumor type, tumor grade, seizure/other neurologic symptoms, duration of symptoms, performance status, extent of surgery performed, and irradiation dose are important prognostic factors.
The strongest prognostic factors for malignant astrocytomas, before irradiation has been given, are age, tumor type, performance status, and extent of surgery (74).
During treatment, frequent attention must be paid to side effects that may influence the patient’s quality of life.
Medications, performance and neurologic status, laboratory values, and the patient’s social situation must be monitored to optimize his or her ability to accept and receive appropriate treatment.
Glucocorticoids (usually dexamethasone) are used preoperatively, postoperatively, and often during the early phases of irradiation to decrease cerebral edema. They should be tapered to the lowest dose necessary to control symptoms; slow decreases in doses are necessary when steroid use is discontinued.
Treatment for the primary tumor may alleviate seizures (if present), but residual CNS injury may continue to predispose to seizures. Concomitant medications (such as glucorticoids) may alter anticonvulsant medication pharmacokinetics (particularly phenytoin); serum anticonvulsant levels should be checked regularly, particularly after medication changes.
In most tumors, maximal safe surgical resection is associated with improved prognosis; in some tumors, complete surgical resection may be curative.
Some chemotherapy agents have demonstrated significant radiosensitizing effects in the CNS (58). In particular, temozolomide, an oral alkylating agent, is an important drug in the treatment of gliomas that can offer potent radiosensitization, which is sometimes manifested as dramatic tumor necrosis after treatment with concurrent irradiation and temozolomide (13).
Radiation therapy can be delivered to the CNS by fractionated external beam radiation therapy, radiosurgery or stereotactic irradiation, or interstitial brachytherapy.
External beam irradiation: Therapy is commonly started 2 to 4 weeks after surgery to allow for wound healing. Current treatment regimens for primary CNS tumors generally include (dependent principally on histology) doses of 45 to 60 Gy (1.8- to 2.0-Gy fractions) with three-dimensional (3-D) conformal irradiation or intensity-modulated radiation therapy (IMRT). Treatment schedules delivering higher doses or using larger fraction sizes (greater than 2 Gy per fraction) are associated with higher risks of late CNS toxicity. In addition to dose considerations, the volume of brain irradiated to high dose must be minimized; this is best accomplished by multiple, cross-firing treatment beams with careful blocking of the uninvolved brain in 3-D conformal irradiation or by use of IMRT. Hyperfractionated and accelerated fractionated schemes have been explored in clinical trials but have not shown a clear benefit (64, 118). For tumors at high risk of spread to the CSF space, elective irradiation of the
whole craniospinal axis, with a localized boost to the area of gross tumor volume (GTV), may be necessary. Matching of orthogonal, diverging beams of radiation when the brain and spinal cord are treated necessitates careful planning and patient immobilization to avoid overdose in junction areas between treatment fields.
Radiosurgery or stereotactic irradiation: Delivery can be achieved with a linear accelerator or specialized equipment (e.g., Gamma Knife, Cyber Knife). Targets typically do not exceed 3 to 4 cm and must be sufficiently distant from critical structures (e.g., optic nerves and brainstem) so that they are not included in the high-dose volume (78). Dose conformality to the target volume is generally an important component of treatment planning.
Brachytherapy: Selection criteria for implant have included the following: tumor confined to one hemisphere; no transcallosal or subependymal spread; size less than 5 to 6 cm; well circumscribed on CT or MRI; and accessible location for implant. Direct infusion of specific radioimmunoglobulins in primary and recurrent brain gliomas has been used (85).
Particles: Proton and charged-particle beam radiation therapy has been used in some intracranial tumors, particularly clivus and base of skull tumors such as chordomas and chondrosarcomas (23, 38, 75, 91).
Radiation modifiers: Phase I and II studies in malignant glioma have suggested a potential benefit to treatment with halogenated pyrimidines during radiation therapy for anaplastic astrocytoma (110). Temozolomide is a potent radiosensitizing alkylating agent that is a component of standard management of high-grade glioma patients; others studies on radiosensitizers for gliomas have focused on agents such as motexafin gadolinium, mammalian target of rapamycin inhibitors, and farnesyltransferase inhibitors (13).
Follow-up: The follow-up schedule must be frequent to monitor side effects and properly taper any steroid medications. The physician must ensure that neurologic status is optimized. Tumor recurrence may be detected with periodic CT or MRI scans. Assessment of intellectual functioning and quality of life is especially important in patients with benign tumors, as well as in long-term survivors with malignant tumors; patients should also be monitored for neuroendocrine and ophthalmologic side effects.
The external acoustic meatus are bilaterally symmetric; they participate in the definition of anatomic reference planes in the head (Reid’s baseline and Frankfort horizontal plane, connecting points in the two external acoustic meatus and one anterior infraorbital edge).
On a lateral projection radiograph, the sella turcica is centrally located and marks the lower border of the median telencephalon and diencephalon. The hypothalamic structures are located an additional 1 cm superior to the sellar floor, and the optic canal runs, at the most, 1 cm superior and 1 cm anterior to that point.
The pineal body (tentorial notch) usually sits approximately 1 cm posterior and 3 cm superior to the external acoustic meatus.
The cribriform plate, the most inferior part of the anterior cranial fossa, is an important reference point for the inferior border of whole-brain irradiation fields; adequate coverage of the cribriform plate is particularly important for conditions with a predilection for recurrences in the frontobasal fossa above the cribriform plate, such as medulloblastoma. In most patients, little distance is found between the lateral projections of the lenses and the most inferior part of the cribriform plate. It may not be possible to both completely block out the lenses and include the cribriform plate in whole-brain irradiation (122).
The head should be positioned so that its major axes are parallel and perpendicular to the central axis incident beam and the treatment table; the most common errors are rotation of the head and longitudinal axis deviation (tilting).
Reproducibility of head positioning is achieved with a fixation device such as the table-fixed thermoplastic net mask, Flixster stereotactic device (table-fixed reference plate attached to a plastic turban plus mouthpiece), or an individually made mouthpiece attached to a table frame.
Whole-brain irradiation is administered through parallel-opposed lateral portals, which should always be individualized.
The inferior field border should be 0.5 to 1.0 cm inferior to the cribriform plate, middle cranial fossa, and foramen magnum, which should be distinguishable on simulation radiographs (2-D planning) or digitally reconstructed radiographs (CT-based 3-D planning).
The anterior border must be about 3 cm posterior to the ipsilateral eyelid for the diverging beam to exclude the contralateral lens; however, this supplies the posterior ocular bulbs with only about 40% of the prescribed dose. A better alternative is to angle the gantry about 5 to 7 degrees anteriorly from true lateral, so that the beam’s anterior border traverses the head in a frontal plane about 0.5 cm posterior to the lenses (about 2 cm posterior to eyelid markers). This arrangement provides full dose to the posterior parts of the ocular globe (46).
For high-grade gliomas, it was initially recommended that large volumes or even the entire intracranial contents should be irradiated because of their diffuse nature. However, in 35 patients who had a CT scan within 2 months of an autopsy, 78% of recurrences were within 2 cm of the margin of the initial tumor bed and 56% were within 1 cm or less of the volume outlined by the CT scan (37). No unifocal tumor recurred as a multifocal lesion. These findings were confirmed by others (118).
In a review of CT scans and pathologic sections of 15 patients with glioblastoma multiforme (GBM), if radiation treatment portals had been designed to cover the contrast-enhancing volume along with a 3-cm margin around the edema, they would have covered all histologically identified tumors in all cases (33).
Relatively generous margins (i.e., 3 cm) and inclusion of all radiographic evidence of tumor and associated edema is generally the rule in designing treatment portals for high-grade gliomas. The classic RTOG method entails irradiating enhancing tumor plus peritumoral edema plus a 2- cm margin to 46 Gy, followed by a boost of 14 Gy to enhancing tumor plus a 2.5- cm margin (110). However, some investigators have reported that it is potentially safe (i.e., no change in failure patterns) not to deliberately use the peritumoral edema region to generate the clinical target volume (CTV) in high-grade glioma irradiation (12).
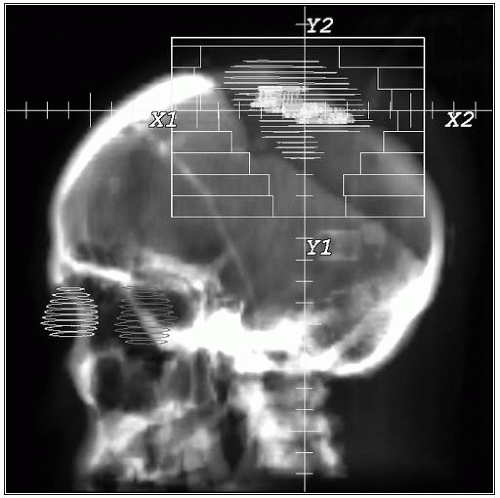
Figure 11-3 illustrates the initial portal used for the treatment of a multifocal anaplastic astrocytoma (WHO grade III) with some brain edema to deliver 45 Gy, and the reduced portal to deliver an additional 14.4- Gy boost.
Bilateral or medial cerebral hemispheric tumors are often best treated with parallel-opposed portals.
If the tumor is asymmetric or lateralized, combinations of dual-photon energies (e.g., 6 and 20 MV) may provide better dose distributions, yielding higher tumor doses and diminishing the dose to the uninvolved normal brain (16).
Frontal lesions encompassing only the anterior parts of the lobe can be treated with anterior and lateral isocentric perpendicular beams; the dose distribution can be optimized with wedges in either or both beams.
Midcerebral tumors (posterior frontal or anterior parietal) may best be treated with parallelopposed anterior and posterior portals and lateral portals, all isocentric and with or without wedges.
Posterior parietal or occipital lesions can be treated with posterior and lateral isocentric beams, both suitably wedged for dose homogenization.
Lesions in the temporal lobe tip may be difficult to treat with other than lateral portals unless the patient is flexible enough to tuck the chin against the chest so that a sagittal beam can be added that does not traverse the optic structures/lenses.
Craniopharyngiomas and pituitary, optic nerve, hypothalamic, and brainstem tumors are deep and centrally located. Depending on the extent, they may be treated—for example—with isocentric three-portal, four-portal, rotation, or arc-rotation treatment techniques. Stationary beams give adequate dose homogeneity in and around the sella turcica. The three-field technique consists of parallel-opposed lateral portals and an anterior vertex portal (i.e., superior anterior oblique). The lateral portals may be wedged to compensate for the declining anteroposterior dose gradient from the anterior portal. The four-field box technique uses both lateral and sagittal parallel-opposed portals. A 360-degree rotation technique can be used if fixation is adequate to avoid geographic misses. Because of the shorter distance from the anterior surface, the cylindrical dose distribution becomes flattened posteriorly. Arc rotation with reversed edges enables an ellipsoid dose distribution.
Brainstem lesions may be adequately treated with parallel-opposed lateral portals combined with a posterior midline portal that does not irradiate the eyes.
Unilateral cerebellar lesions also can be covered by appropriately wedged posterior and lateral portals.
Pineal lesions can often be treated with parallel-opposed lateral portals.
Superficial lesions (e.g., superior sagittal sinus meningiomas) can be treated with parallelopposed isocentric tangential fields or half-beam block technique to avoid inferior divergence of beams into the normal brain.
3-D conformal therapy (3-DCRT) has supplanted 2-D irradiation techniques in the treatment of primary and metastatic brain tumors.
When International Commission on Radiation Units Report No. 50 (40) is used for 3-D treatment planning, the GTV encompasses the enhancing tumor (and surrounding edema for select histologic types) on CT or MRI scans. The CTV adds 1 to 3 cm, depending on histologic type/grade. The planning target volume (PTV) adds 0.5 to 1.0 cm.
Multiple planar and noncoplanar fields encompassing the tumor (and surrounding edema for some tumors) with appropriate margin (PTV) are used—sometimes with static or dynamic wedges and multileaf collimation—to deliver the therapeutic dose. The treated volume should generally be encompassed by at least the 95% isodose volume.
Marks et al. (63) described some of these techniques; they reported that noncoplanar beams may be preferable to coplanar beams when the target is in the central regions of the head.
Sometimes the CT scan defines abnormalities not always perceptible on MRI studies. Integration of MRI and CT scan data may be necessary for optimal 3-D treatment planning of brain tumors. This can be achieved through computerized CT-MRI registration functions available in many commercial treatment planning software platforms.
Brain tumors that generally require craniospinal irradiation of the CNS and the entire subarachnoid space (neuraxis) include medulloblastoma, supratentorial PNETs, disseminated ependymoma, pineoblastoma, disseminated germ cell tumors, and other CNS tumors with dissemination/metastases.
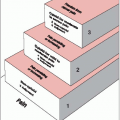
The patient is typically irradiated with boost, whole-brain (“helmet”), and spine fields. Generally, the entire craniospinal axis is initially irradiated (whole-brain and spine fields), followed by treatment with boost fields to the tumor itself (plus appropriate margin as needed).
The boost is an individualized portal arrangement that depends on tumor size and location. Multiple portals (with wedges as necessary) or rotational fields may be used; 3-DCRT and more recently IMRT are delivery techniques commonly employed for boost irradiation.
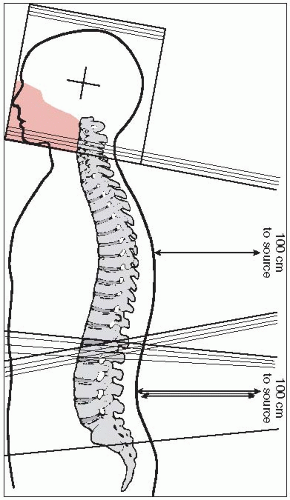
For craniospinal irradiation, the patient is simulated prone with thermoplastic mask immobilization and with the neck hyperextended to minimize posteroanterior (PA) spinal field exit through the mandible/oral cavity (but not so hyperextended as to cause excessive posterior neck skin creasing and compromise in dose uniformity for the spinal field).
Spinalfield(s): The spine fields are usually simulated first. In adults, the spinal fields are commonly one superior and one inferior field (whereas in children, the entire spinal subarchanoid space can often be covered with one field, especially with the use of an extended source-tosurface distance [SSD] technique such as SSD = 120 to 130 cm) (Fig. 11-4). The superior spinal field has a stationary central ray location, with its initial superior edge typically immediately above the level of the shoulders. This superior border is then usually moved cranially (junction shift) by about 1 cm after each 9 Gy (i.e., five 1.8-Gy fractions) is delivered, with the inferior border of the whole-brain fields appropriately moved cranially by 1 cm as well. Although no gap between the whole-brain and superior spinal light fields is needed if appropriate collimator rotation of the whole-brain fields is performed (see below), many institutions prefer adding an approximately 0.5 cm safety gap between the whole-brain and superior spinal fields. The inferior border of the superior spinal field has a skin gap with the superior border of the inferior spinal field such that these edges of the two fields do not overlap within the spinal cord (i.e., calculation of the gap such that the field edges intersect at anterior spinal cord/posterior vertebral body corpus). After each 9 Gy, this junction between spine fields is either moved superiorly or inferiorly (depending on institutional convention) by about 1 cm; however, if at all possible, this junction between spinal fields should always be below the level of the true cord (in adults, generally cord ends at L1). The inferior border of the inferior spinal field must cover the thecal sac (often at S2) with appropriate margin of about 2 cm; a sagittal MRI view should be used to determine the termination of the thecal sac. The central ray for the inferior spinal
field is moved with the moving junction between the two spinal fields because the caudal border of the inferior spinal field is fixed; however, the central ray location for the inferior spinal field is fixed if using a modern asymmetric jaw technique in order to change the length of the inferior spinal field that lies above the central ray axis. The spinal field width(s) should be adjusted so that the lateral field borders are at least 1 cm lateral to the lateral edge of each ipsilateral pedicle; most institutions recommend a widening of the inferior spinal field width at its inferior end by about 1 to 2 cm to cover the more widely spaced sacral nerve roots, although the need to extend out to the sacroiliac joints is controversial (“spade” field design) (32).
Whole-brain fields: Parallel-opposed whole-brain lateral fields are simulated with the central ray approximately in the pineal region. The inferior field border is allowed to reach the most inferior cervical vertebra (usually around C4-5) without traversing the ipsilateral shoulder. When the whole-brain/spinal field junctions are moved, the whole-brain field can be conveniently decreased without a change in the position of the isocenter. The gantry can be angled up from the horizontal position for each whole-brain field (until markers placed on the two lateral orbital canthi align) so that each whole-brain field avoids the contralateral lens. Although this technique allows the ocular bulb behind the lens to reach full-dose levels (46), it risks underdosing the cribriform plate region. Blocks are drawn on the whole-brain fields so that the irradiated volume includes the olfactory groove (cribriform plate), the orbits 3 cm posterior to the eyelid markers (2 cm if gantry is angled anteriorly from true lateral), the middle cranial fossa plus about 1 cm margin, and the included cervical vertebral bodies (Fig. 11-4). As described above, the junction between the whole-brain and superior spinal fields is usually gapped about 0.5 cm, with this junction usually moved cranially by approximately 1 cm after every 9 Gy delivered.
To match the divergence of the superior spinal field anteriorly, a collimator rotation is applied to whole-brain fields (Fig. 11-4).
One method to avoid caudal divergence of whole-brain fields into the superior spinal field is to rotate the foot of the couch toward the gantry for each of the whole-brain fields.
Primary chemotherapy is rarely used as the sole treatment for intracerebral malignancies.
Nitrosoureas, vincristine, procarbazine, cisplatin, and carboplatin have been used in conjunction with surgery and irradiation.
The current standard of care for the treatment of high-grade gliomas after tumor resection or biopsy is concurrent chemoirradiation with use of the oral alkylating agent temozolomide, followed by adjuvant temozolomide: 5-year overall survival in the landmark EORTC/NCIC Phase III trial of 9.8% with concurrent temozolomide plus irradiation followed by up to six cycles of adjuvant temozolomide versus 1.9% with irradiation alone (104). Methylation of the promoter of MGMT (O6-methylguanine DNA methyltransferase), a DNA repair gene, strongly predicts benefit from temozolomide (35, 104).
Newer agents such as bevacizumab (an antiangiogenic agent) have also been employed; bevacizumab as monotherapy and bevacizumab/irinotecan have yielded some promising results in the recurrent high-grade glioma setting (19, 54, 114, 131).
The addition of PCV (procarbazine, CCNU, vincristine) to irradiation has demonstrated improved progression-free survival (but not overall survival) in the treatment of anaplastic oligodendroglioma (7, 113). Tumors with chromosome 1p and 19q co-deletions appear to be associated with improved survival and greater chemosensitivity to PCV and potentially also to temozolomide (even as monotherapy without irradiation) (72, 83).
For tumors with leptomeningeal spread or at high risk of CSF involvement, direct intrathecal injection of chemotherapeutic agents into the CSF space has been used. A limited number of agents (thiotepa, methotrexate, cytosine arabinoside) are suitable for intrathecal injections (57). Some novel agents that have been delivered intrathecally for select cases include liposomal cytarabine, rituximab (for lymphoid malignancies), and trastuzumab (for breast cancer).
Nausea and vomiting independent of changes in intracranial pressure may occur, particularly with posterior fossa or brainstem irradiation.
Radiation dermatitis is usually mild and may be treated with topical petrolatum, lanolin, or hydrocortisone.
Alopecia within the irradiated area may be permanent with higher total doses.
Otitis externa may occur if the ear is included in the irradiation fields; serous otitis media also may occur.
Inclusion of the inner ear may be associated with high-tone hearing loss and, occasionally, vestibular damage.
Mucositis, esophagitis, and diarrhea may develop with craniospinal irradiation due to the exit of the spinal fields through the oropharynx, mediastinum, and abdomen.
Fatigue may occur and blood counts may decrease during treatment, particularly with largevolume cranial or craniospinal irradiation.
In the 6 to 12 weeks after irradiation, neurologic deterioration may occur as a subacute side effect (somnolence syndrome). This is attributed to changes in capillary permeability and transient demyelination secondary to damage to oligodendroglial cells.
The most serious late reaction to irradiation is radiation necrosis, which may appear 6 months to many years (peak at 3 years) after treatment; it can mimic recurrent tumor. Management of symptomatic radiation necrosis may include surgical debulking in combination with steroid treatment. Focal necrosis is more often a result of irradiation alone, whereas a more diffuse leukoencephalopathy is more commonly seen after combined treatment with chemotherapy (particularly methotrexate) and irradiation.
Inclusion of the eye may lead to retinopathy or cataract formation. Optic chiasm and nerve injury may cause a general decrease in visual acuity, visual field changes, or blindness at doses greater than 50 to 54 Gy.
Onset of hormone insufficiency from irradiation of the hypothalamic-pituitary axis is variable but may be seen with doses as low as 20 Gy (especially growth hormone [GH] deficiency).
Cranial irradiation can produce neuropsychological changes such as decreased learning ability, deficits in short-term memory, and difficulties with problem solving, particularly in older adults and after whole-brain irradiation.
A summary of treatment recommendations for selected malignancies is given in Table 11-2.
Treatment of anaplastic astrocytoma and glioblastoma is similar. Current recommendations call for maximal safe surgical resection (or biopsy if no resection possible) followed by adjuvant irradiation with concurrent and adjuvant temozolomide (104).
Because malignant gliomas are infiltrative, even gross total resection inevitably results in tumor recurrence.
Localized irradiation volumes generally encompass either the peritumoral edema with a 2- to 3- cm margin, the contrast-enhanced volume (plus any resection cavity) with an approximately 3- cm margin, or the RTOG technique of enhancing tumor plus peritumoral edema with a 2 cm margin to 46 Gy followed by a boost of 14 Gy to enhancing tumor (plus any resection cavity) with a 2.5- cm margin (110).
TABLE 11-2 General Treatment Recommendations for Selected CNS Malignancies | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Attempts at radiation dose escalation, often with use of hyperfractionation or stereotactic boost techniques, have failed to demonstrate consistent survival benefit for high-grade glioma patients.
For patients with poor pretreatment prognostic factors and limited expected survival, palliative treatment (30 Gy in 10 fractions in 2 weeks) may provide adequate symptom control without excessively protracted treatment.
A regimen of 40 Gy in 15 fractions has been shown to be noninferior to the standard 60 Gy in 30 fractions for glioblastoma patients aged 60 years or older (86).
Even for elderly patients, radiotherapy has been shown to provide a survival benefit without a reduction in quality of life or cognition when compared with supportive care measures alone (47).
Postchemoirradiation development of “pseudoprogression” (treatment effect-related apparent tumor progression on imaging such as increasing contrast enhancement and/or necrosis in the immediate postchemoirradiation period, which subsequently stabilizes or wanes with further follow-up) occurs in approximately 20% of patients receiving temozolomide with irradiation, appears to be more common in patients with tumor MGMT methylation, and may be associated with improved survival (4, 5).
The relative rarity of these lesions, absence of randomized trials, reliance on institutional retrospective reviews, and long natural history make it difficult to make dogmatic treatment recommendations (120).
Pilocytic astrocytomas are more amenable to total resection than other low-grade gliomas. In solid neoplasms, resection of the contrast-enhancing portion is sufficient.
Fenestration of the cyst and resection of the mural nodule are usually curative.
In completely resected pilocytic astrocytomas, no adjuvant therapy is needed.
Postoperative irradiation in subtotally resected pilocytic astrocytoma may be appropriate depending on symptoms, extent of residual disease, availability for follow-up, and feasibility of repeat surgical excision.
Chemotherapy (e.g., carboplatin/vincristine) is often used as first-line therapy for optichypothalamic tumors.
If radiation therapy is indicated, 50.4 to 54.0 Gy (1.8-Gy fractions) usually is sufficient.
Although observation is reasonable for select patients, treatment is often instituted for patients with increasing tumor size, progressive/uncontrolled neurologic symptoms (e.g., refractory seizures), or evidence of malignant transformation.
The treatment regimen is generally maximal safe resection (biopsy if no resection possible), followed by adjuvant radiotherapy. However, immediate (rather than salvage) postoperative irradiation is associated with a progression-free survival benefit, not overall survival benefit (112). Postoperative irradiation is often deferred in patients with complete resection who are 40 years of age or younger.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree