OECD and EPA differ in the parameters they prefer for conducting the uterotrophic assay, including the route of exposure and which animal model to use. Several factors should be considered when selecting a route of exposure for the uterotrophic assay, including the relevant route of exposure to the test compound, bioavailability by the oral route, and metabolism to an active or inactive metabolite. The typical route of administration for the uterotrophic assay is either oral gavage or sc injection. OECD TG states that the most relevant route of exposure should be used with consideration to avoid first pass metabolism [8], whereas EPA TG favors sc dosing for direct entry of a compound into the general circulation, thereby avoiding gut metabolism and slowing the rate of liver metabolism [9]. The sc route may warrant consideration if the compound has a positive in vitro estrogen receptor (ER) binding assay, particularly if uterotrophic results by the oral route were negative. These data may be useful to characterize hazard but may be of little relevance if the germane exposure to a compound is via the oral route and the substance is rapidly deactivated by first-pass hepatic metabolism. For many compounds, the route of exposure does not impact the ability to detect a positive uterotrophic response if dose levels are selected appropriately, although the minimum effective dose may be an order of magnitude higher with oral gavage compared with sc injection [16]. Of course, the precise difference between effective sc and oral doses depends on the specific compound in question.
When selecting the animal model, OECD favors the immature female rat, whereas EPA prefers the ovariectomized young adult model. Both the immature and ovariectomized adult models are viewed as equivalent for reliability and assay sensitivity [18,10]. There are advantages and disadvantages to each animal model, which should be considered prior to model selection. The immature model may be preferred for animal welfare concerns as surgery is avoided; however, careful planning is needed to avoid the use of excess numbers of animals (i.e., due to the young age of the immature females, litters of animals must be ordered, yet dams and male littermates are not needed for this assay). If the immature model is selected, female rats must be used prior to puberty and the corresponding increase in endogenous estrogen production (i.e., postnatal day [PND] 18–25 with necropsy no later than PND 25; PND 0 is defined as the day of birth). This coincides with the period of maximum sensitivity of the uterus to exogenous estrogens [19–21]. Due to the need to necropsy these animals by PND 25, the dosing period is limited with the immature model, whereas the dosing period can be extended with the ovariectomized adult model if desired. Furthermore, immature rats are more sensitive to dietary phytoestrogen content due to the consumption of greater amounts of feed per kilogram of body weight, and uterine weights are more sensitive to body weight-mediated effects in this model, which increases the need to carefully consider group body weights during the study. The immature model is slightly less specific than the adult ovariectomized model; positive uterotrophic results with the immature model can be caused by interaction of a test substance with the hypothalamic-pituitary-gonadal (HPG) axis (e.g., aromatizable androgens can be positive in this assay).
If the ovariectomized model is selected, animals must undergo surgery, then be allowed time for the uterus to regress (i.e., approximately two weeks). During this interval, uterine weights will decrease markedly; however, these weights may not reach a stable baseline [22,23]. In addition, incomplete ovariectomy can lead to marked increases in uterine weights [24]; thus, vaginal smears are included over the last five days of the recovery period to verify that ovariectomy was complete, and laboratory personnel must look for ovarian remnants at necropsy if this animal model is used. One advantage of the ovariectomized adult is increased specificity for compounds that interact with the ER compared to the immature model. The adult ovariectomized model allows for a longer dosing period if desired; assay responsiveness for some weak estrogenic compounds (e.g., o,p′-DDT) improved with seven days of sc dosing, although assay sensitivity (i.e., the ability to detect a positive response with a true estrogenic compound) was not affected with either three or seven days of dosing [25].
In addition to the control and treated groups, a positive control group exposed daily to 17α-ethynylestradiol (EE) may be included in the assay to verify assay sensitivity and performance. In fact, OECD and EPA TGs require the inclusion of an EE-treated positive control group or a study demonstrating laboratory proficiency by generating a dose-response curve for EE-induced uterotrophic responses when conducting uterotrophic assays for regulatory purposes. If a positive control group is included, the EE dose should approximately equal the effective dose (ED) inducing a 70 to 80 percent increase in uterine weight relative to the maximum uterine weight increases induced with EE in the dose-response study (i.e., ED70 or ED80). Inclusion of an EE positive control group allows the laboratory to confirm responsiveness of the assays relative to previous historical control data to verify that there are no shifts in assay sensitivity.
To ensure assay sensitivity, steps should be taken to verify that baseline uterine weights are not artificially elevated due to alternate sources of estrogens. Consequently, test animals are given a low phytoestrogen rodent diet (genistein equivalents ≤350 μg/g diet), as higher phytoestrogen content may increase baseline uterine weights [16]. Criteria for acceptable baseline uterine weights have been defined by both OECD and EPA. OECD states that baseline uterine weights generally range from 20 to 35 mg for immature rats and 80 to 110 mg in the ovariectomized young adults [16]. EPA states that the mean blotted uterine weight for the vehicle control group should be <0.04 percent of terminal body weight (ovariectomized model) or <0.09 percent of terminal body weight (immature model) to yield sufficient assay sensitivity [9]. Furthermore, immature blotted uterine weights of 40 to 45 mg in the control group may warrant concern for assay sensitivity, and weights greater than 45 mg may require the assay to be rerun in the event of a negative or equivocal result. Thus, these baseline data, coupled with EE response data, indicate that the assay is performing as expected and would detect treatment-related increases in uterine weight if these increases were present.
Variability also is an area of concern. If variability in control uterine weights is excessive, the ability of the uterotrophic assay to detect weakly acting estrogenic compounds will be diminished. In OECD validation study [10], statistical power calculations for the uterotrophic assay are presented; for example, there was reasonable power (81 percent probability) of detecting a 35 percent increase in uterine weight with six animals per group if the coefficients of variation (CVs) remained low (i.e., 15.0 percent). Blotted uterine weights generally have less variability than imbibed uterine weights [10]. Each laboratory should establish its own historical control data for baseline uterine weights to determine whether uterine weights in the vehicle control group are atypically high, and a repeat of the assay should be considered.
In the uterotrophic assay, issues with reproducibility may arise when increases in uterine weights are in the lower portion of the dose-response curve and, therefore, may or may not achieve statistical significance [16] and/or baseline uterine weights are outside the normal range, altering assay sensitivity. Careful dose selection, using range-finding studies if needed, and evaluation of baseline uterine weights within the context of historical control data can help clarify uterotrophic results. Suspect results may warrant assay replication. Thus, modest increases in uterine weight should be interpreted carefully using a WoE approach.
While relatively specific for estrogenic compounds, the uterotrophic assay also may yield a positive response with androgens, progestins, and other growth factors [6,26,27]. While measurement of uterine weight increases has been the most consistent and sensitive indicator of estrogenic activity [28], uterine histopathology may confirm an estrogenic response (e.g., testosterone can increase uterine weight but produces different histopathology from estrogen [19]). In addition, data from ER binding assays, ER transactivation assays, or other related end points (e.g., estrogen-sensitive molecular markers) may help confirm estrogenicity [29]. Results of previous toxicity studies also should be reviewed for signs of estrogen perturbations.
While not included as a requirement of the EDSP, the estrogen antagonist portion of the uterotrophic assay also can be used to detect anti-estrogens by measuring a test compound-mediated attenuation in uterine weight increases when co-administered with estrogen [7,30]. OECD has issued a guidance document for laboratories interested in using the uterotrophic assay to test for anti-estrogenicity [31]. However, most environmental agents with estrogenic activity are mixed agonists/antagonists [10] and can be detected in the uterotrophic assay without including the anti-estrogenic portion of the assay. With the anti-estrogenic assay, it is important to note that the induction of liver enzymes by a test compound may enhance the rate of estradiol clearance and reduce the increases in uterine weight [32–35] via a non-ER-mediated mechanism.
11.2.2 Hershberger Assay
The rodent Hershberger assay is a short-term, in vivo Tier 1 endocrine screening assay designed to detect androgenic or anti-androgenic activity by measuring a compound’s ability to produce an increase in accessory sex tissue (AST) weights or inhibit the testosterone-induced increase in AST weights in castrated (orchidoepididyectomized) peripubertal rats. AST weights are androgen dependent (i.e., testosterone and dihydrotestosterone [DHT]); thus, the Hershberger assay was designed to detect chemicals that potentially act as androgen receptor (AR) agonists, antagonists, or 5α-reductase inhibitors [36–39] (5a-reductase converts testosterone to DHT). Because animals are castrated, the HPG axis is disrupted, so the assay does not detect agents that act directly on the hypothalamus or pituitary or via a non–receptor-mediated mode of action (e.g., altered steroidogenesis). The Hershberger assay is included in EPA’s EDSP, and TGs are available that describe the conduct, interpretation, and performance specifications for this assay [40,41].
As with the uterotrophic assay, the Hershberger assay underwent an extensive validation program coordinated by OECD [15,38,39,42–45]. The Hershberger assay has been shown to reliably detect androgenic and anti-androgenic activity across numerous laboratories using different routes of exposure in castrated rats. Overall, the assay shows good reproducibility both within and between laboratories and relatively good specificity (e.g., see Tables 4 and 5 in [39]). The following discussion is focused primarily on the conduct and utility of the Hershberger assay for EDSP screening.
To conduct the Hershberger assay (see Figure 11.2), male rats are castrated at 42 days of age or shortly thereafter and allowed 7 days to recover from surgery. During this time, the ASTs regress. Castrated male rats (6/dose group) receive test material for 10 days by gavage or sc injection in the presence or absence of testosterone propionate (TP). EPA TG for the Hershberger assay specifies a limit dose of 1,000 mg/kg/day [41]. If the limit dose approach is not applicable, a minimum of two treatment groups are required for the androgenic portion of the assay and three treatment groups for the anti-androgenic portion of the assay. Additional treatment groups can be included if needed. As with the uterotrophic assay, the test material may be given either orally or subcutaneously, depending on the relevant route (oral, dermal, or inhalation), toxicological considerations, and the desire to avoid first-pass metabolism. Animals are euthanized 24 hours after the final dose, and organ weights are collected for the ASTs (i.e., ventral prostate, seminal vesicles with coagulating glands and fluid, levator ani-bulbocavernosus muscles, glans penis (if preputial separation has occurred), Cowper’s (bulbourethral) glands), and other organs (i.e., liver, kidneys, and adrenals). Compounds that increase AST weights in the absence of TP are considered positive for androgenic activity, whereas compounds that decrease AST weights in the presence of TP are considered positive for anti-androgenicity. On the designated necropsy day, blood may be collected for possible future measurement of serum testosterone, luteinizing hormone (LH), or follicle stimulating hormone (FSH). Significant alterations in two or more AST weights are required for a positive assay outcome.
FIGURE 11.2 Schematic drawing of the Hershberger assay design (see the text for study design details). The inset shows a testosterone-stimulated increase in seminal vesicle weights (top) and illustrates the relative size of accessory sex tissues (LABC, Cowper’s glands, seminal vesicles) in non-androgen-treated castrated adult rats (bottom). The lowest tissue shown in the bottom photo is the LABC from a testosterone-treated male.
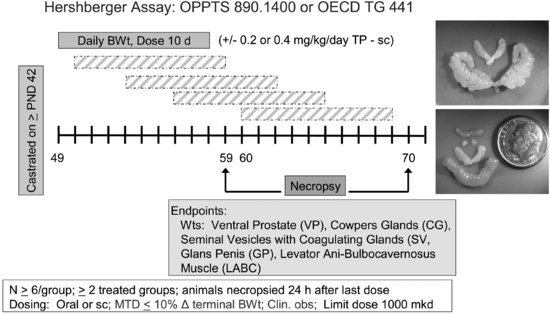
During the validation work, numerous factors were examined for effects on assay specificity. The assay is not sensitive to rat (mouse) strain used, diet, bedding, caging, light cycles, or animal room conditions (temperature, humidity) as long as the animals in a particular study are maintained under the same conditions [36,38]. Furthermore, the assay is relatively insensitive to body weight-mediated changes in AST weights [46].
Because the Hershberger assay can detect both androgenic and anti-androgenic responses, concurrent controls are an important component of the assay protocol. For the androgenic portion of the assay, animals given 0.2 or 0.4 mg/kg/day TP sc are included as a positive control for comparison with vehicle control animals to verify sensitivity to androgenic responses. For the anti-androgenic portion of the assay, animals are given a combination of 3 mg/kg/day flutamide orally and TP subcutaneously as a positive control for comparison with the TP-only animals to verify sensitivity to anti-androgenic responses. Thus, for each assay, a vehicle control, positive androgenic control (TP), and a positive anti-androgenic control (flutamide with TP) are needed along with groups exposed to the test compound with and without TP.
In the Hershberger assay, as with the uterotrophic assay, a number of nonrequired optional end points may be measured, including liver, kidney, and adrenal weights, and hormone levels (testosterone, LH, FSH, triiodothyronine [T3], and thyroxine [T4]). Liver, kidney, and adrenal weights can provide additional information on systemic toxicity and/or metabolic enzyme induction. Measurement of testosterone, LH, and FSH may provide additional information about mode of action. Some investigators have assessed thyroid weight and/or histopathology as part of the Hershberger assay [47–49]. The value of measuring thyroid hormone (TH) levels is questionable because TSH levels are not measured, and results may be very difficult to interpret in the absence of thyroid weights and thyroid histology, which are not measured in this assay. Furthermore, the sample sizes specified in the Hershberger assay (n = 6) are generally considered too small for accurate assessment of TH levels due to variability among animals; thus, increasing group size should be considered if these optional end points are to be included. In general, the optional end points that have the greatest potential for enhancing assay interpretation are liver weights and, if warranted, hepatic enzyme evaluation [7].
While not technically difficult, the Hershberger assay requires some practice to conduct consistently. Dissection of AST tissues from animals not exposed to TP requires some practice as these tissues are very small (e.g., paired Cowper’s gland weights are often 5–6 mg). Due to the small size, variability may increase in these end points, which may confound assay results [50]. In addition, there may be a limited sample size for glans penis weights if all animals have not undergone preputial separation. If male Sprague-Dawley-derived rats are castrated at 42 days of age and the mean age for preputial separation is 41.8 to 45.9 days of age [51], some animals may not have completed preputial separation at the time of the assay. Laboratories are required to report and statistically analyze the number of animals that failed to complete preputial separation in the control and treated groups; however, given the 7-day recovery period after castration (i.e., animals are at least 49 days of age at the time of dosing), it is questionable whether differences in preputial separation can be attributed to treatment. Thus, differences in the number of animals achieving preputial separation, and therefore sample sizes for glans penis weights, do not necessarily reflect androgenic or anti-androgenic potential of the test material.
While EPA and OECD provide performance criteria for assay results, laboratories must be cautious in their use of those criteria. Per the TGs, CVs for the control and high-dose groups must meet the performance criteria when assay outcomes are negative; if the maximum CV criteria have been exceeded, the assay may lack sufficient sensitivity to detect AST weight changes. Therefore, the assay may need to be repeated in the event of a negative or equivocal finding. CV criteria are generally applied to the control group; it would not be unusual for the high-dose group to have greater variance than the controls due to greater interanimal variability in the response to test material treatment. High CVs can result in the need to repeat the assay. To assist interpretation, laboratories should maintain historical control data for vehicle controls, testosterone-treated controls, and testosterone-plus-flutamide-treated controls to verify that assay results are consistent with previous findings.
Within the various AST tissues, there are differential responses to androgens, which can provide some information on mode of action. For example, the weight of the ventral prostate is more sensitive to DHT than to testosterone and so is more reflective of 5α-reductase activity. In contrast, the size of the levator ani muscle is testosterone dependent, with little capacity to convert testosterone to DHT, thereby serving as a more specific marker of anabolic (myotrophic) androgens. With the 5α-reductase inhibitor finasteride, effects on ventral prostate weights were seen at lower doses than those affecting levator ani-bulbocaveronosus muscle weights [52]. Although differences in response magnitude may occur across tissues, AST weights should exhibit a trend in the same direction [39]. Thus, differential effects on organ weights may provide some clues about a chemical’s mode of action; however, additional data would be required for a definitive determination.
It is important to use a WoE when evaluating Hershberger assay results because the weights of the target tissues may be altered by agents other than androgen agonists or antagonists (e.g., estrogens can increase seminal vesicle weights). Aside from testosterone and DHT, AST weights can be altered by TH, growth hormone, prolactin, and/or epithelial growth factor as well as estrogens.
Compounds that induce hepatic enzymes may enhance the rate of testosterone clearance and thereby produce a positive anti-androgenic response without interaction with either ARs or 5α-reductase. This possibility was recognized during the assay validation process; hence, the inclusion of liver weights and optional histopathology as part of the Hershberger assay. One example of this possibility, identified in our laboratory, involves a dinitroaniline herbicide. When tested in a Hershberger assay, this herbicide markedly increased liver weights and significantly decreased AST weights in testosterone-treated animals. Analysis of serum hormone samples collected 24 hours after the last dose indicated that testosterone levels were decreased 29 percent with compound treatment. A subsequent clearance study indicated that treated animals eliminated 14C-testosterone in the blood four times faster than control animals, resulting in plasma area under the curve (AUC) values significantly lower than the controls after 10 days of treatment. Previous toxicity studies have demonstrated that this compound activates enzymes involved in thyroid and steroid hormone clearance. Furthermore, the available data do not support anti-androgenicity via altered 5α-reductase activity or AR binding. When comparing organ weight decrements in the Hershberger study, the levator ani-bulbocavernosus muscle was more sensitive than ventral prostate, which suggests that an effect on 5α-reductase activity is less likely. Furthermore, this dinitroaniline herbicide was negative for AR binding at concentrations up to 1,000 μM, as demonstrated using a competitive binding assay with ARs from rat prostate cytosols (M. LeBaron, personal communication). Thus, these data suggest that enzyme-inducing compounds can produce positive Hershberger responses through a mode of action that does not directly involve the endocrine system. Optional end points (e.g., liver weights/histopathology and serum testosterone levels in terminal blood samples) can aid in determining the mode of action for anti-androgenic responses. Another alternate (or complementary) approach is to examine hepatic enzyme induction directly [37,53]. After collecting organ weights, the liver can be frozen for possible enzyme evaluation if anti-androgenic effects are observed. It also may be useful to monitor testosterone clearance in satellite animals when hepatic enzyme induction is suspected.
While the Hersbherger assay is relatively specific for detecting compounds interacting with the AR or inhibiting 5α-reductase, it is still possible to obtain positive results by other modes of action aside than these. Thus, this assay, as with all of the Tier 1 EDSP assays, should be used in a WoE approach that considers all available toxicity data and the results of other Tier 1 assays, particularly results for AR binding, the male pubertal assay, and the fish short-term reproduction assay.
11.2.3 Male and Female Pubertal Assays
The male and female pubertal assays are longer term (21–31 days), repeat-dose in vivo assays designed to detect potential estrogenic/anti-estrogenic effects (primarily the female assay), androgen/anti-androgen effects (primarily the male assay), steroid biosynthesis inhibitors, alterations in the HPG axis, and thyroid perturbations by evaluating a compound’s ability to alter age at puberty onset, estrous cycles (female only), or reproductive/AST/thyroid weights or histopathology. Serum hormone measurements for testosterone (males only), T4, and TSH are included to provide additional information on possible endocrine activity. One of the strengths of the pubertal assays is that endocrine end points are examined in young animals during a dynamic period when integrated function of the endocrine system is required. These assays are multimodal, capable of detecting endocrine-active chemicals that operate through a variety of modes of action, some of which are not detected by in vitro, uterotrophic, or Hershberger components of the EDSP. Thus, the male and female pubertal assays are included in EPA’s EDSP Tier 1 assays. TGs are available that describe the conduct, interpretation, and performance specifications for these assays [54,55].
The male and female pubertal assays underwent a validation program coordinated by EPA [56,57]; however, the validation work for these assays was not as extensive and did not include as many laboratories as the uterotrophic or Hershberger validation efforts. Overall, the male and female pubertal assays have been shown to reliably detect multiple endocrine modes of action. For endocrine-active compounds used in the validation program, the overall pattern of effects was reproducible across laboratories, although sometimes effects on specific end points were not reproducible. Furthermore, these assays showed good sensitivity to detect endocrine-active compounds, but there is some concern that these assays may lack specificity due to their reliance on apical end points. The next discussion is focused primarily on the conduct and utility of the pubertal assays for EDSP screening.
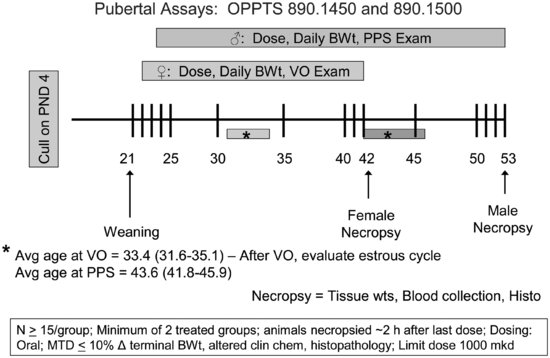
To conduct the male and female pubertal assays (see Figure 11.3), male or female weanling rats are randomly assigned to treatment groups in a manner that yields similar mean body weights and variances across groups; littermates are not assigned to the same group. Rats are exposed to the test compound by oral gavage from PND 23 to 53 (males) or 22 to 42 (females). Beginning on PND 30 (males) or PND 22 (females), animals are evaluated daily for puberty onset, which is indicated by preputial separation in the males and vaginal opening in the females. When puberty onset is achieved, the animal’s age and body weight are recorded. Once vaginal opening is complete, daily vaginal smears are collected from female rats to monitor age at first estrus and to evaluate the pattern and regularity of the estrous cycle. Males and females are necropsied on PND 53 and 42, respectively. A terminal blood sample is collected for clinical chemistry and serum hormone analyses (TSH and T4 in both the males and females and testosterone in the males). The liver, kidneys, adrenals, thyroid, and pituitary are weighed in both sexes. Gender-specific organ weights include: ovaries, and uterus (with and without fluid) in the females and testes, epididymides, ventral prostate, dorsolateral prostate, seminal vesicles with coagulating glands (with and without fluid), and levator ani-bulbocavernosus muscles in the males. Tissues examined histopathologically include the ovary, uterus, kidney, and thyroid for the females and the testis, epididymis, kidney, and thyroid for the males. Many of the end points evaluated in the pubertal assays (e.g., puberty onset, estrous cyclicity, some organ weights, and histopathology) are redundant end points with the EDSP Tier 2 multigeneration rat reproduction study, which allows confirmation of these screening results under more robust test conditions.
FIGURE 11.3 Schematic drawing of the male and female pubertal assay designs (see the text for study design details). VO = vaginal opening; PPS = preputial separation; MTD = maximum tolerated dose.
The inclusion of numerous apical end points in the male and female pubertal assays creates greater inherent biological variability in the end points evaluated and may contribute to difficulties in identifying the mode of action for endocrine-active compounds. For example, in EPA’s prevalidation and validation studies, mean age at preputial separation in control animals varied from 39.6 to 43.9 days of age and mean age at vaginal opening in control animals ranged from 31.5 to 34.9 days of age. The basis for this interanimal variability in age at puberty onset is poorly understood, although puberty onset can be influenced by a number of factors, including alterations in higher brain function [58–60], growth hormone [61], melatonin [62], body weight/composition [63], animal husbandry practices [64–66], and interlaboratory differences in recording these landmarks. Similarly, estrous cycle length is variable across animals, particularly with the onset of cycling, and it is subject to influence by body weight gain and stress [67,68]. In the female pubertal assay, estrous cycle must be monitored after vaginal opening; however, the duration of time for estrous cycle monitoring (∼ 10 days if vaginal opening occurs at 32 days of age) is limited, particularly when one considers that females have four- to five-day cycles and that the first days after vaginal opening may be acyclic. In one of the prevalidation studies conducted for the female pubertal assay, 12 of 14 control animals failed to achieve regular cycles during the monitoring period after vaginal opening [56]. Furthermore, organ weights can be affected by multiple endocrine modes of action, by the stage of estrous cycle at necropsy, or by nonspecific effects, such as systemic toxicity or stress, which can cause changes in body weight gain. Thus, given that multiple factors can affect end points like puberty onset, estrous cyclicity, and organ weights, examining patterns of effects across end points is more useful than examining individual end points when determining a chemical’s mode of action.
Results with known endocrine-active compounds have raised questions regarding the sensitivity of the pubertal assays and their ability to differentiate modes of action. With respect to sensitivity (i.e., the proportion of active substances that are correctly identified by a new test), the available data indicate that the male and female pubertal assays are relatively sensitive with respect to alterations in estrogen and androgen function. However, there remains some question as to the ability of the female pubertal assay to detect weak aromatase inhibitors, particularly those with mixed endocrine activities. The female pubertal assay did not detect δ-testolactone, a moderately specific aromatase inhibitor, at doses high enough to cause anti-androgenicity in the male pubertal assay [69]. The Integrated Summary Report for the female pubertal assay [56] notes that minimal effects were seen with the weak aromatase inhibitor fenarimol at doses that produced a significant decrease in terminal body weight (9.8 percent). Ultimately, fenarimol was detected as an endocrine-active compound due to thyroid changes, which would complicate mode of action determinations. In contrast, the potent aromatase inhibitor fadrozole was readily detected in the female pubertal assay [69].
Similar issues were reported for the male pubertal assay with respect to some weak thyroid-active compounds. Phenobarbital was used as a weak thyroid agent during validation of several EDSP assays; however, phenobarbital did not alter thyroid weights, thyroid histopathology, or serum T4 or TSH levels in the male pubertal assay [57]. Instead, phenobarbital delayed preputial separation and decreased reproductive and AST weights, likely via a central nervous system effect. Therefore, phenobarbital produced the same pattern of effects as the anti-androgens linuron and flutamide. If phenobarbital was an unknown compound, it is unclear whether it would have been identified as a primary thyroid toxicant when a variety of anti-androgenic effects were seen at lower dose levels.
Specificity also has been recognized as a potential issue for the male and female pubertal assays. The Interagency Coordinating Committee on the Validation of Alternative Methods defines “specificity” as the proportion of inactive substances that are correctly identified. Initially, 2-chloronitrobenzene was used as a negative control chemical for the male and female pubertal assay validation; however, it was later determined that this was a poor choice for a negative control compound as multiple end points were altered in these assays [56,57]. EPA recently reported that hydroxyatrazine (OH-ATR) and 2,4-dichlorophenoxyacetic acid (2,4-D) were negative in the male and female pubertal assays [70]. The maximum tolerated doses (MTDs) for both OH-ATR and 2,4-D were identified through renal toxicity rather than a significant change in body weight/body weight gains. For OH-ATR, renal toxicity seen at 45 mg/kg/day included pyelonephritis (inflammatory of the renal pelvis), whereas for 2,4-D, renal toxicity (3 and 30 mg/kg/day) was based on minimal to slight renal changes (mineralization, tubular regeneration, and the presence of protein casts). Thus, the renal changes caused by OH-ATR and 2,4-D span a spectrum of severity. With only a few negative compounds included in the pubertal assay validation, the question of assay specificity remains open and may be further examined as additional data are developed.
The negative control examples further emphasize the importance of careful selection of the high dose level (MTD) when conducting the male and female pubertal assays. According to the pubertal TGs, these dose criteria may constitute an MTD:
MTD dose selection for the pubertal assays is critical to avoid nonspecific outcomes. Thus, a relatively inclusive range-finding study may be useful to select dose levels.
Male and female pubertal assay results may be difficult to interpret in the presence of changes in growth rate and terminal body weight. Interpreting delays in puberty onset can be problematic in studies where endocrine-mediated effects must be distinguished from generalized delays in growth. While Laws et al. [71] reported that 20 to 21 percent decreases in body weight did not significantly affect age at puberty onset in male or female rats, other data suggest that age at puberty onset and body weight function as a continuum [72], and body weight alterations of approximately 10 to 15 percent could alter puberty onset [73,74]. Organ weight end points also may be affected by changes in body weight. Feed restriction studies have demonstrated that female organ weights were not altered with a 5 percent change in terminal body weight; however, the next level of feed restriction (12 percent) altered pituitary, adrenal, liver, kidney, and ovarian weights [71]. In a separate study, a 8.6 percent change in terminal body weight altered female pituitary and kidney weights and the number of four- to five-day estrous cycles [75]. Similar results have been reported in the male pubertal assay. Terminal body weight decreases of 4 percent altered adrenal and liver weights in the males, whereas adrenal, pituitary, liver, and kidney weights were altered at the next level of feed restriction (12.5 percent) [71]. Marty et al. [46] reported that an 11 percent decrease in terminal body weight altered epididymidal, prostate, ventral prostate, seminal vesicle, and liver weights, and a reanalysis of these data indicated a significant decrease in dorsolateral prostate weights as well. These findings are similar to feed restriction results reported by Stoker et al. [73], wherein a 15 percent difference in terminal body weight resulted in significant decreases in ventral prostate, seminal vesicle, and epididymal weights. There are no data as to whether the weights of the levator ani-bulbocavernosus muscles and seminal vesicles without fluid are influenced by body weight changes as neither organ weight has been measured in pubertal feed restriction studies. Data also indicate that TH levels can be altered with feed restriction [71]. Although the precise magnitude of body weight change that results in significant differences in assay end points is unclear, both male and female pubertal assay results must be interpreted with caution if a 10 percent change in terminal body weight is observed. EPA has recognized this point; for the male pubertal assay, the TG cautions that a 6 percent decrease in terminal body weight should be interpreted with caution using a WoE approach and in these cases, additional studies may be needed to determine endocrine activity. According to the female pubertal TG, terminal body weight decreases up to 10 percent are consistent with the MTD description.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree