Existing data and nontest information
All available (eco)toxicological data from standardized or nonstandardized tests
Read across, chemical categories, (Q)SARs and other in silico predictions, and ADME model predictions
In vitro assays providing data about selected endocrine mechanism(s)/pathways(s) (mammalian and nonmammalian methods)
Estrogen receptor transcriptional activation (OECD TG 455)
Androgen or thyroid transcriptional activation (If/when TGs are available)
Steroidogenesis in vitro (OECD TG 456)
MCF-7 cell proliferation assays (ER ant/agonist)
Other assays as appropriate
In vivo assays providing data about selected endocrine mechanism(s)/pathway(s)1
Hershberger assay (OECD TG 441)
Amphibian metamorphosis assay (OECD TG 231)
Fish Reproductive Screening Assay (OECD TG 229)
Fish Screening Assay (OECD TG 230)
Androgenized female stickleback screen (GD 140)
In vivo assays providing data on adverse effects on endocrine-relevant end points2
Repeated dose 90-day study (OECD TG 408)
First-generation assay (OECD TG 415)
Male pubertal assay (see GD 150)3
Female pubertal assay (see GD 150)3
Intact adult male endocrine screening assay (see GD 150)
Prenatal developmental toxicity study (OECD TG 414)
Fish Reproduction Partial Life Cycle Test (when/if TG is Available)
Larval Amphibian Growth and Development Assay (when TG is available)
Avian Reproduction Assay (TG 206)
Mollusc Partial Life Cycle Assays (when TG is available)4
Reproductive screening test (OECD TG 421 if enhanced)
Combined 28-day/reproductive screening assay (OECD TG 422 if enhanced)
Developmental neurotoxicity (TG 426)
In vivo assays providing more comprehensive data on adverse effects on endocrine relevant end points over more extensive parts of the life cycle of the organism2
Two -generation assay (OECD TG 416 most recent update)
Medaka Multigeneration Test (MMGT) (when TG is available)
Avian two-generation reproductive toxicity assay (when TG is available)
Mysid Life Cycle Toxicity Test (when TG is available)4
Copepod Reproduction and Development Test (when TG is available)4
Sediment Water Chironomid Life Cycle Toxicity Test (TG 233)4
Mollusc Full Life Cycle Assays (when TG is available)4
Daphnia Reproduction Test (with male induction) (OECD TG 211)4
Daphnia Multigeneration Assay (if TG is available)4
Explanatory notes for Table 12.1:
1Some assays may also provide some evidence of adverse effects.
2Effects can be sensitive to more than one mechanism and may be due to non-ED mechanisms.
3Depending on the guideline/protocol used, the fact that a substance may interact with a hormone system in these assays does not necessarily mean that when the substance is used, it will cause adverse effects in humans or ecological systems.
4At present, the available invertebrate assays solely involve apical end points that are able to respond to some EDs and some non-EDs (those in Level 4 are partial life cycle tests, while those in Level 5 are full or multiple life cycle tests).
5The new EOGRT study (OECD TG 443) is preferable for detecting endocrine disruption because it provides an evaluation of a number of endocrine end points in the juvenile and adult F1, which are not included in the second-generation study (OECD TG 416) adopted in 2001.1
12.2 OVERVIEW OF THE OECD REVISED CF
As agreed initially in 2002 and in the 2011 update, the OECD CF agreed is not a testing scheme but rather represents a pragmatic hierarchy in which the various tests that can contribute information for the detection of the hazards of endocrine disruption are placed. The tool box was originally organized into five levels, each corresponding to a different level of biological complexity for both human health (mammalian) and ecological (nonmammalian) areas. The OECD CF is also not an endocrine disrupter testing strategy. Importantly, any test at any level can be conducted as questions arise, so that it is not necessary to follow the CF in a rigid or linear manner. Instead, the CF should be used flexibly depending on the nature of existing information and the needs for testing and assessment. Entering is possible at all five levels and depends on the nature of existing information and needs for testing and assessment. Also, the assessment of each chemical should be on a case-by-case basis, taking into account all available information, bearing in mind the function of the framework levels. Briefly, examples of information that can be collated at the different levels of the CF are:
In order to illustrate the principles that are used here to underpin the application of the OECD CF for analysis of assay data, two case studies are presented in this chapter. We have used two well-studied endocrine-disrupting chemicals with different modes of action, from two difference usage classes: the pharmaceutical 17α-ethynylestradiol (EE2) and the agrochemical vinclozolin (VIN). EE2 is an estrogen receptor (ER) agonist while VIN is an androgen receptor (AR) antagonist. Importantly, given the huge amount of information available on the endocrine activity of these chemicals in diverse animal models, only examples of data can be included within the scope of this chapter. Therefore, the examples given should not be used per se to make conclusions about the overall hazard or risk assessment profile of either EE2 or VIN to human health or to the environment. Furthermore, consideration is largely restricted to studies with mammals and aquatic organisms.
12.3 APPLICATION OF THE KLIMISCH CRITERIA TO THE EE2 AND VIN CASE STUDIES
For the purposes of this chapter, the authors adopted the recommendations of Klimisch et al. [9] for reviewing (eco)toxicology data from various sources. Such an approach is often helpful so that different reviewers consistently assess data with a common approach to reliability, relevance, and adequacy (e.g., provision of data on measured test concentration in fish studies as opposed to only nominal concentrations, which are less reliable). A summary of the approach used in the current exercise is given in Table 12.2. This approach to rating studies according to their reliability enables a reviewer to focus the assessment on key studies and improves clarity of the assessment and conclusions. In the future, it is hoped that some of the uncertainties that can arise in published papers on (eco)toxicology experiments will be reduced through the adoption of improved animal usage documentation under the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines [10].
Table 12.2 Klimisch checklist as a guide for study reviews [9]
| K | Methods | Data/Information |
| 1 | Guideline study (OECD, ISO etc.) | In Vivo Test animals (species, strain, sex, age) Purity/composition/origin of the test substance Number of animals evaluated Scope of the investigations per animal and methods description Description of the changes/lesions observed Control group or historical control data of the laboratory Description of the test conditions Description of the route and doses of administration Dose/concentration relationship In Vitro Description of the test system and test method in details Purity/composition/origin of the test substance Dose/concentration differentiated according to the toxicity of test substance on the test system; information on volatility Data on secondary effects that may influence a result (e.g. solubility, impurities, pH shifts, etc.) Appropriate –ve/+ve controls as integral parts of the test References on adequacy of method given/generally known |
| Comparable to guideline study | ||
| Procedure according to national standards (e.g., ASTM, DIN, EPA etc.) | ||
| 2 | Well-documented and meets basic scientific principles | |
| Basic data given: comparable to guidelines/standards | ||
| Comparable to guideline study with acceptable restrictions | ||
| 3 | Method not validated | |
| Documentation insufficient for assessment | ||
| Does not meet important criteria of current methods | ||
| Relevant method deficiencies—unsuitable test system | ||
| 4 | Only short abstract available | |
| Only secondary literature (review, tables, books, etc.). | ||
| K = Klimisch score | ||
12.4 CASE STUDY: DATA EXAMPLES FOR 17α-ETHYNYLESTRADIOL
In a human health context, EE2 has been intensively studied in the process of drug development using preclinical animal models and through wider medical and family planning studies. Hence the in vitro (see Table 12.3) and in vivo mammalian (see Table 12.4) data cited in this chapter are a tiny fraction of the data published; nonetheless, they illustrate how such studies can be evaluated in the context of the OECD CF. Likewise, since a number of studies showed the dramatic impacts of EE2 on fish reproduction at very low concentrations (nanogram per liter levels), this has led to a relatively large number of ecotoxicology studies that are beyond the scope of this chapter. For example, an August 2011 search on Google Scholar using the terms “fish and ethynylestradiol” gave >5,800 hits while “environment and ethynylestradiol” gave 15,000 hits; hence, in this instance, the reader is referred to Caldwell et al. [11] for a review of EE2 and its impact on aquatic species. Most important, our emphasis is on illustrating the principles of the OECD CF for nonmammalian species (see Table 12.5) rather than a definitive review of all the published data.
Table 12.3 2011 OECD Revised Conceptual Framework: Level 1 and 2 data examples for 17α-ethynylestradiol (EE2)
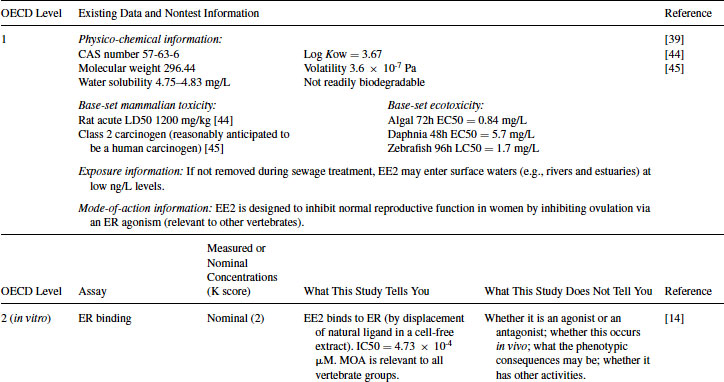
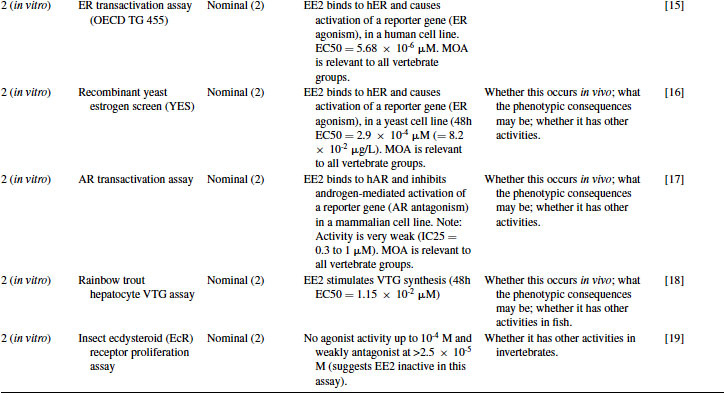
Table 12.4 2011 OECD Revised Conceptual Framework: Levels 3 to 5 data examples on mammalian toxicology studies with EE2
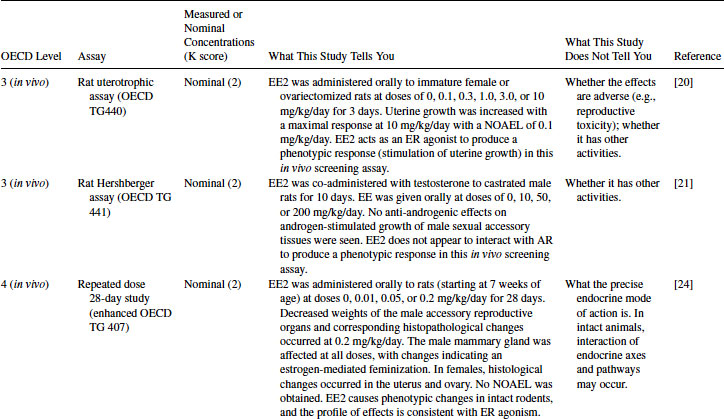
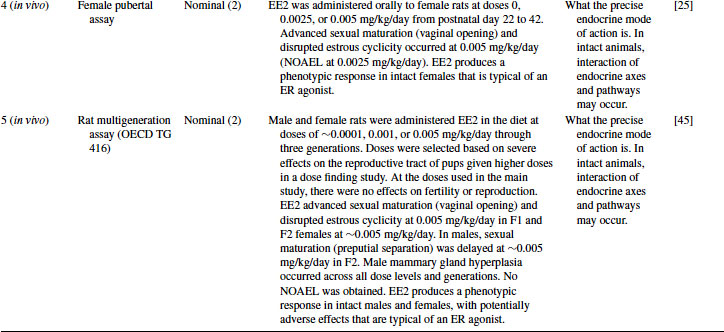
Table 12.5 2011 OECD Revised Conceptual Framework: Levels 3 to 5 data examples on nonmammalian toxicology studies with EE2
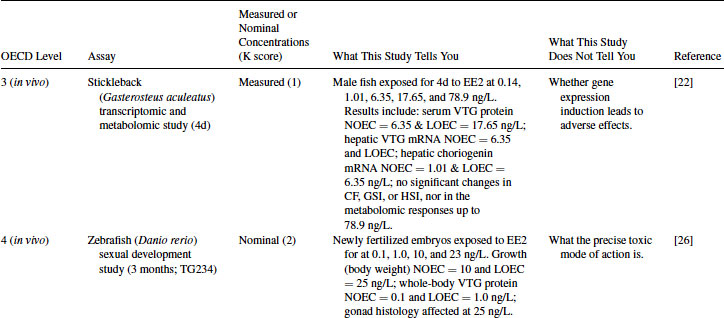
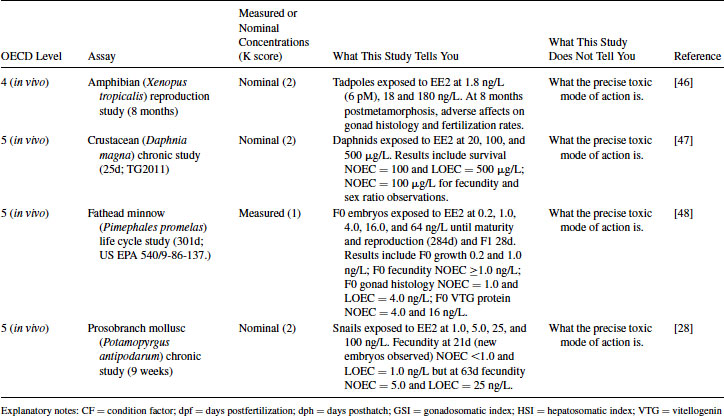
12.4.1 EE2 Case Study: Level 1 Information
EE2 (CAS number 57-63-6) is a semisynthetic alkylated oestradiol with a 17α-ethinyl substitution. It has high estrogenic potency in mammals when administered orally and is often used as the oestrogenic component in oral contraceptives (http://drugbank.ca/drugs/DB00977). The physico-chemical properties of EE2 and exposure information are shown in Table 12.3. Aside from the longer-term studies summarized elsewhere in this chapter, human pharmaceuticals traditionally have been tested for their acute toxicity in rodents and other preclinical models. The U.S. Food and Drug Administration [12] also requires such compounds to be tested in acute ecotoxicity studies using algae, crustaceans, and fish. These data, together with other available information are summarized in Table 12.3.
12.4.2 EE2 Case Study: Level 2 Information
EE2 has been tested in a wide variety of in vitro assays developed for drug discovery purposes by the pharmaceutical industry, including the recombinant hER yeast-based assay developed by Glaxo and then given to university researchers in the mid-1990s to aid ecotoxicology research [13]. It has also been tested in more recently developed assays, including OECD Test Guideline (TG) 455 (ER transactivation assay for estrogen agonists). As shown in Table 12.3, EE2 binds to the ER in a competitive binding assay [14] with a high degree of potency, similar to that of the natural estrogen estradiol. This assay, however, cannot distinguish between agonists and antagonists. Transactivation assays are therefore necessary to make this distinction, and EE2 acted as an agonist in both a mammalian hER cell line (OECD TG 455) [15] and the yeast hER cell line [16] with a high degree of potency. In contrast, although EE2 had some activity in an AR transactivation assay [17], this was considered to be very weak compared to anti-androgens such as R1881. ER agonism is therefore the most important mode of action of EE2. Given the highly conserved nature of ER, this is relevant across vertebrate taxa. Importantly, however, such studies do not provide information on whether there would be any expressed endocrine-mediated activity in biological tissues. These in vitro assays also do not take into account adsorption and metabolism of the chemical in whole animals (considered in Levels 3–5). As another example, EE2 has been tested by Pelissero et al. [18] and shown to dramatically induce the production of vitellogenin (VTG) in fish hepatocytes in vitro. Finally, evidence of the absence of in vitro activity in a given assay can be informative, as demonstrated by the use of an arthropod cell line [19], which showed EE2 to be almost inactive against the ecdysteroid receptor (see Table 12.3). Although this last assay is not a test guideline and is not relevant to vertebrate hormone systems, it provides data on the potential of a chemical to interact with an arthropod receptor and provides information complementary to the OECD in vivo chronic arthropod test guidelines (e.g., using chironomids or daphnids). All examples cited here have been rated at Klimisch code 2, since the biological aspects of the work are clearly described, but the precise chemical concentrations used were not verified by analytical chemistry (a typical approach within in vitro toxicology).
12.4.3 EE2 Case Study: Level 3 Information
EE2 has been tested in the rat uterotrophic assay for estrogen agonists. In the example given in Table 12.4, it was positive in both immature and ovariectomized rat assays as uterine weight was significantly increased [20]. The positive result in this assay corresponds well with the positive results obtained in the Level 2 ER-based in vitro assays. A no-observable-adverse-effect level (NOAEL) of 0.1 mg/kg/day was achieved when EE2 was orally administered (the most relevant route), although assays at this level are designed to be in vivo screening assays, providing only a positive or negative answer. The result confirms that EE2 acts as an ER agonist in vivo, but the uterotrophic assay cannot provide definitive information about whether the effects are adverse. The uterotrophic assay has been designed to be sensitive. The ovariectomized assay does not possess an intact hypothalamus-pituitary-gonad (HPG) axis, and the assay will largely detect only estrogenic and anti-estrogenic chemicals. Adverse effects on reproduction and development are not predicted. Likewise, if other modes of action occur, they are unlikely to be detected in this assay.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree