Summary of Key Points
- •
Development of malignant mesothelioma is usually associated with asbestos exposure.
- •
The most common genetic alterations in mesothelioma are deletion of CDKN2A/ARF , inactivation of NF2 , and mutation or deletion in BAP1.
- •
The eighth edition of the American Joint Commission on Cancer/Union for International Cancer Control staging manual has altered the T and N components for staging from the previous edition.
- •
Clinical, imaging, and serum biomarkers can contribute to prognostication in pleural mesothelioma.
- •
First-line palliative chemotherapy is cisplatin (or carboplatin) with pemetrexed; appropriate patients may benefit from the addition of bevacizumab.
- •
There is no standard second-line chemotherapy for pleural mesothelioma although reintroduction of a pemetrexed-containing regimen, or the use of vinorelbine or gemcitabine, would be considered reasonable.
- •
Optimal surgical management of malignant pleural mesothelioma remains controversial.
- •
Immunotherapies including mesothelin-targeted agents and immune checkpoint inhibitors have shown promising efficacy in mesothelioma but further research is required before these are adopted as standard therapy.
All mesothelioma tumors originate from the lining of the pleural cavity, the lung, the pericardium, and the abdominal cavity, including the tunica vaginalis. After transformation of the mesothelium or peritoneum, subtypes of mesothelioma can develop. Malignant mesothelioma (MM) occurs most frequently on one side of the thorax (Malignant Pleural Mesothelioma [MPM]—80%) with the remainder occurring in the abdomen as malignant peritoneal mesothelioma. Although the World Health Organization classification distinguishes three main histologic subtypes of mesothelioma, epithelioid (60%), sarcomatoid (10% to 15%), and biphasic (25% to 30%), there are different subtypes of the epithelioid histology. These subtypes include the papillary, pleomorphic, tubulopapillary, and small cell type. However, these subtypes are not standardly reported.
The main cause of MPM is the exposure to asbestos fibers, which was first described by Wagner et al. Other causes of mesothelioma are endemic erionite exposure in Turkey, ionizing radiation, and chronic inflammation in the pleura. Unlike the case with lung cancer, cigarette smoking does not play a role in the development of MPM. MPM is one of the best-known occupational diseases and it is more likely to develop in men than in women (90% vs. 10%), primarily as a result of its association with mining and processing of asbestos fibers. Given the long latency period of 30 to 50 years, the prevalence of mesothelioma is expected to peak for the next decade. Regulations against handling and mining asbestos in Western Europe and the United States were put in place in the 1990s. It is expected that the disease will be increasingly encountered in third-world countries because of the lack of legislation and increased export to these countries.
Biology of Malignant Pleural Mesothelioma
Considerable progress has been made in understanding the molecular basis of mesothelioma, which has, in turn, led to an abundance of preclinical studies translating these discoveries to treatment.
Some people with mesothelioma have no history of asbestos exposure or prior radiotherapy. In the case of peritoneal mesothelioma, many patients are teenagers or young adults. Good evidence now exists that at least in some people there may be a genetic basis for developing mesothelioma, which could lead to mesothelioma by itself or cause some individuals to be susceptible to asbestos carcinogenesis. Cyclin-dependent kinase inhibitor 2A/alternative reading frame (CDKN2A/ARF) , neurofibromatosis type 2 (NF2) , and BRCA1-associated protein 1 (BAP1) are the most frequently mutated tumor suppressor genes in mesothelioma.
Cyclin-Dependent Kinase Inhibitor 2A/Alternative Reading Frame
CDKN2A/ARF is the most frequently inactivated tumor suppressor gene in malignant mesothelioma and encodes two important cell cycle regulatory proteins, p16 (INK4A) and, in an alternative reading frame, p14 (ARF) . p16, a cyclin-dependent kinase (CDK) inhibitor, blocks phosphorylation of retinoblastoma protein, and p14 (ARF) blocks murine double minute 2 (MDM2) , thus resulting in a positive regulation of p53. Homozygous deletion of CDKN2A/ARF thus results in inactivation of two major tumor suppressing pathways, retinoblastoma protein and p53.
CDKN2A deletion is found in about 70% of primary tumors and nearly all mesothelioma cell lines.
Neurofibromatosis Type 2
The NF2 gene encodes a tumor suppressor protein Merlin, a member of the band 4.1 family of cytoskeletal linker proteins. Inactivating NF2 mutations are found in 35% to 40% of MPMs. The mechanisms of Merlin-mediated tumor suppression are not well defined. Merlin mediates contact-dependent inhibition of cell proliferation in normal cells, primarily through inhibition of mammalian target of rapamycin (mTOR) in an AKT-independent manner. mTOR activity is aberrantly upregulated in the absence of Merlin, leading to increased cell proliferation.
BAP1
Up to 60% of mesotheliomas have BAP1 alterations, which include, among others, homozygous deletions of partial or entire BAP1 and sequence-level mutations. BAP1 is located on chromosome 3p21.1, which is also deleted in several human malignancies. Germline BAP1 mutations were described in 2011 in mesothelioma families in which BAP1 mutation carriers had an exceptionally high incidence of malignancies, including mesothelioma and uveal melanoma. These malignancies did not develop in family members who did not carry germline BAP1 mutations. BAP1 is a nuclear protein that enhances BRCA1-mediated inhibition of breast cancer cell proliferation, acting as a tumor suppressor in the BRCA1 growth control pathway and regulating proliferation by deubiquitinating host cell factor. BAP1 influences a wide array of cellular functions, and its depletion induces significant changes in the expression of many genes that control various cellular pathways.
Diagnosis
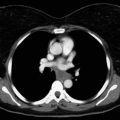
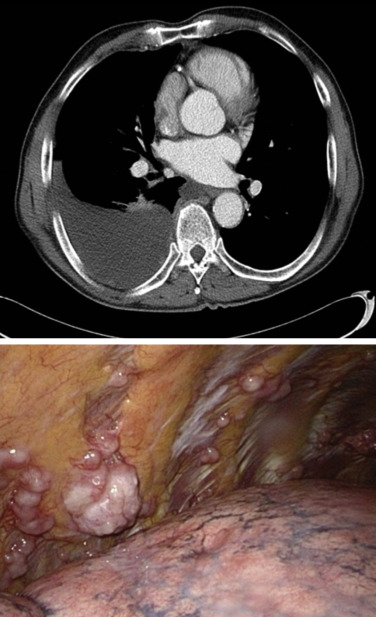
Diagnosis of mesothelioma may be achieved by cytologic analysis of pleural effusions sampled by thoracentesis, blind pleural biopsy, computed tomography (CT)-directed fine-needle aspiration or core biopsy, and, increasingly more commonly, via pleuroscopy. However, in a considerable number of cases, evaluations of specimens obtained by these less invasive modalities are inconclusive, necessitating surgical biopsy. Video-assisted thoracoscopic surgery (VATS) is the preferred method for surgical diagnosis of MPM ( Fig. 53.1 ). VATS allows large tissue samples to be obtained from multiple areas of the thoracic cavity, an important consideration, given the considerable tumor heterogeneity within individual mesothelioma tumors. Multiple separate biopsies by VATS increases the likelihood of accurately determining histologic subtype. VATS can also be used to identify whether the tumor involves the visceral pleura as well as the parietal pleura, although this distinction is no longer required for staging with the updated American Joint Commission on Cancer (AJCC)/Union for International Cancer Control (UICC) eighth edition guidelines. VATS is otherwise of limited use for assessment of tumor or node stage. Although VATS is most easily performed in patients with a large effusive component, occasionally tumor burden is such that VATS is impossible due to fusion of the visceral and parietal pleurae and, in such instances, a small, 2-cm cutdown can usually allow access to the underlying pleural tumor under direct vision. A further merit of VATS is that it allows talc pleurodesis to be done at the time of tissue diagnosis, which often obviates the need for additional palliative procedures. Pleurodesis does not influence the ability to perform subsequent cytoreductive surgery (pleurectomy/decortication [PD] or extrapleural pneumonectomy [EPP]). Talc will cause fluorodeoxyglucose (FDG) activity in the pleural distribution and in mediastinal lymph nodes on positron emission tomography (PET) imaging. For this reason, initial staging with PET should be performed before talc pleurodesis.
Thoracotomy is to be avoided as a diagnostic method as it not only causes the patient unnecessary trauma but also hampers the performance of subsequent cytoreductive surgery due to disruption of tissue planes and risks iatrogenic tumor invasion of the chest wall.
Staging
The AJCC/International Mesothelioma Interest Group staging system is based primarily on pathologic data and is, therefore, of limited use when applied to clinical staging of this disease. Many of the factors that contribute to stage assignment, such as involvement of the pericardium, lung, and diaphragm; involvement of the endothoracic fascia; and lymph node metastases, for example, simply are not possible to determine accurately with current imaging technology. The staging system has recently undergone revision based on an international multicenter prospective data collection of detailed staging information and component descriptors ( Table 53.1 ). The revision for the eighth edition AJCC/UICC staging system has made a number of important changes, including collapsing T1a and T1b into T1 and revising nodal staging such that any ipsilateral mediastinal involved lymph nodes are all included as N1 disease, whereas nodes previously categorized as N3 and reclassified as N2. PET is useful for identifying occult distant metastatic disease (present in up to 25% of cases), but it is inaccurate for determining T and N stage.
| Stage | Definition |
|---|---|
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| TO | No evidence of primary tumor |
| T1 | Tumor imited to the ipsilateral parietal± visceral ± mediastinal± diaphragmatic pleura |
| T2 | Tumorinvolving each of theipsi ateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features:
|
| T3 | Describeslocally advanced but potentially resectable tumor. Tumor involving all of theipsilaterat pleuralsurfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features:
|
| T4 | Describes locally advanced technically unresectable tumor. Tumor involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features:
|
| Regional Lymph Nodes (N) | |
|---|---|
| NX | Regional lymph nodes cannot be assessed |
| NO | No regional lymph node metastases |
| N1 | Metastases in the ipsilateral bronchoputmonary, hilar, or mediastinal (including the internal mammary, peridiaphragmatic, pericardial fat pad, or intercostal lymph nodes) lymph nodes |
| N2 | Metastases in the contralateral mediastinal, ipsilaterat, or contralateral supraclavicutar lymph nodes |
| Distant Metastasis (M) | |
|---|---|
| MO | No distant metastasis |
| Ml | Distant metastasis present |
| IASLC Mesothelioma Staging Project: Stage Grouping Changes | ||||||
|---|---|---|---|---|---|---|
| NO | N1 /N2 | N1 | N3 | N2 | ||
| Stage | Seventh Edition | Eighth Edition | Seventh Edition | Eighth Edition | Seventh Edition | Eighth Edition |
| Tl | I (A, B) | IA | Ill | IV | IIIB | |
| T2 | II | IB | III | IV | IIIB | |
| T3 | IB | Ill | lllA | IV | IIIB | |
| T4 | IV | IIIB | IV | IIIB | IV | IIIB |
| M1 | IV | IV | IV | IV | IV | IV |
∗ Modified from Rusch VW, Chansky K, Kindler HL, Nowak AK, Pass HI, Rice DC, et al. IASLC Staging and Prognostic Factors Committee, advisory boards, and participating institutions.The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol. 2016;11(12):2112-2119.
Laparoscopy and Thoracoscopy
Extension of the tumor through the diaphragm into the peritoneal cavity or dissemination to the contralateral side can occur, which will influence the staging and treatment options. Rice et al. reported on 109 patients who had routine laparoscopy before planned EPP and found nine (8.3%) patients with transdiaphragmatic extension of tumor and one (0.9%) with diffuse peritoneal carcinomatosis. Alvarez et al. performed thoracoscopic examination of the contralateral side and found contralateral chest involvement in three (10%) of 30 patients from a selected group.
Mediastinal Evaluation
Lymph node metastases occur in approximately 50% of patients with MPM undergoing multimodality therapy and portends a poor prognosis. Current imaging modalities are inaccurate for defining N stage, and, therefore, cervical mediastinoscopy has been advocated for pretreatment staging of MPM. The sensitivity, specificity, and accuracy for cervical mediastinoscopy have been reported by two groups and vary from 60% to 80%, 71% to 100%, and 67% to 93%, respectively.
Endobronchial ultrasound (EBUS)- and endoscopic ultrasound (EUS)-guided fine-needle aspiration of mediastinal lymph nodes have been highly effective for staging nonsmall cell lung cancer. Rice et al. compared 50 consecutive patients with mesothelioma who had staging cervical mediastinoscopy with 38 patients who had staging EBUS. Sensitivity and negative-predictive value were 28% and 49%, respectively, for cervical mediastinoscopy, and 59% and 57%, respectively, for EBUS. Furthermore, 11 patients had EUS preoperatively, and metastases were found in the infradiaphragmatic nodes in five patients. Tournoy et al. performed EUS and fine-needle aspiration in 32 patients with presumed early-stage mesothelioma and identified N2 metastases in four patients (12.5%). In 17 patients who subsequently had extrapleural pneumonectomy, one false-negative result (4.7%) was found. Some centers now prefer to stage the mediastinum by combined EBUC and EUS before entering a patient into a multimodality trial.
Biomarkers
Biomarkers have the potential to play a key role in current oncology practice. They can be used in diagnosis (screening), measurement of response, and follow-up. The requisites that apply for biomarkers are technical reproducibility, validation, and clinical relevance. Diagnostic biomarkers can underpin screening programs and early detection in high-risk individuals, can help to direct diagnostic procedures, and can provide support for cytologic or histologic diagnoses. Biomarkers can be predictive for a response or can be prognostic. Predictive biomarkers can assist with treatment selection, in particular drug therapy. Biomarkers of response can accelerate drug development through surrogate end point and provide guidance during routine patient care. Prognostic biomarkers can give patients and clinicians valuable information, in addition to their use as stratification factors in clinical trials. Biomarkers can involve blood measurements (plasma or serum) or molecular genetics or can be based on imaging.
Diagnostic Biomarkers
The development of robust diagnostic serum biomarkers for mesothelioma is important, as exposure to the etiologic agent is often known. A population with heavy asbestos exposure would be rational participants in a screening program. The availability of a blood-based biomarker would facilitate early detection and treatment. The most important candidate diagnostic serum biomarkers are mesothelin (serum mesothelin-related protein), osteopontin, and fibulin-3. Elevated levels of mesothelin are highly specific, unless patients have concurrent renal failure, and add to the diagnostic certainty or direct additional investigations when a diagnosis of mesothelioma is suspected. However, mesothelin lacks sensitivity at the time of diagnosis, thus limiting its use in screening. Osteopontin does not perform as well as mesothelin. In 2012, Pass et al. reported on fibulin-3 as a blood and effusion biomarker. Plasma levels of fibulin-3 were significantly higher for patients with mesothelioma than in people exposed to asbestos without mesothelioma, with a sensitivity of 100% and specificity of 94% reported. Effusion levels of fibulin-3 were also significantly higher in effusions from mesothelioma than from other etiologies; nevertheless, these findings should be further validated before translation to practice. Carcinoembryogenic antigen is a well-known marker that will not be elevated in case of a MPM. It can be used for quick screening for other tumor types with pleural dissemination.
Prognostic Biomarkers
A number of simple prognostic biomarkers have been well established in the literature for MPM. At pathologic diagnosis, a diagnosis of sarcomatoid or nonepithelioid subtypes of mesothelioma is uniformly associated with poor prognosis. The same large retrospective series, mostly based on collections of clinical trial data, has also validated readily available laboratory parameters, including low hemoglobin, thrombocytosis, high white blood cell count, and elevated serum lactate dehydrogenase level as poor prognostic indicators. In 2010, Kao et al. proposed and independently validated an elevated neutrophil-to-lymphocyte ratio as a poor prognostic indicator; however, the patient groups used were heterogeneous and relatively small, and others have been unable to confirm these findings. High serum vascular endothelial growth factor (VEGF) levels have been found to correlate with poor prognosis and advanced stage of disease. A recent publication used a Classification and Regression Tree analysis to group patients into prognostic categories at diagnosis, deriving four prognostic groupings with median survivals ranging from 7.5 months (risk group 4) to 34 months (risk group 1) using readily available clinical indicators including weight loss, hemoglobin level, performance status, histology, and albumin level.
Considering prognostic serum biomarkers more specific to mesothelioma, mesothelin levels at diagnosis may also be prognostic; however, mesothelin levels appear to reflect tumor bulk, as the addition of tumor bulk metrics to the model eliminates the significance of serum mesothelin. No strong evidence exists that serum osteopontin is useful in prognostication, although lower tissue expression of osteopontin may be associated with longer survival and plasma osteopontin as well as mesothelin were found to increase prognostic accuracy in combination with the EORTC and CALGB mesothleioma prognostic indices.
Many candidate tumor molecular and histologic prognostic markers have been reported (phosphatase and tensin homolog, VEGF expression, fibroblast growth factor 2, cyclooxygenase-2, platelet-derived growth factor, epidermal growth factor receptor, epithelial-to-mesenchymal transition, osteopontin, and c-MET expression); however, at the time of publication, none has been sufficiently validated to be entered into routine clinical use worldwide. The anticipation is that the extensive molecular profiling and characterization efforts proceeding worldwide will identify new molecular prognostic biomarkers that will translate to routine clinical practice in addition to providing potential molecular targets for therapy.
Prognostic Imaging Biomarkers
In addition to providing anatomic staging information regarding the sites of disease involvement, a substantial body of evidence indicates that the bulk of tumor as demonstrated on imaging and metabolic characteristics of tumor may be prognostic biomarkers. Tumor volume as measured by CT is a prognostic indicator, but it can be challenging to implement automated volumetric measurements due to the difficulty of distinguishing tumor from pleural fluid and atelectasis. Quantitative parameters from FDG-PET may be simpler to implement reproducibly and with less manual input. FDG-PET has consistently shown prognostic value in MPM, although the appropriate metric for quantitative assessment remains the subject of debate. A higher maximum standardized uptake value (SUV) is a poor prognostic indicator. However, the inclusion of volumetric parameters may also be important, with the concepts of total lesion glycolysis or total glycolytic volume incorporating both SUV and a measure of lesion volume and performing better than SUV alone in studies incorporating both measures.
Chemotherapy
First-Line Chemotherapy
Combination cytotoxic chemotherapy remains the mainstay of palliative treatment of patients with MPM, with cisplatin and an antifolate being the most widely used and evidence-based first-line doublet. The authors of two studies reported that the combination of cisplatin and an antifolate produced a survival benefit over cisplatin alone, with a hazard ratio for overall survival of 0.77 and a median survival gain of 3 months. Cisplatin and pemetrexed have become the standard of care and the backbone on which subsequent clinical trials in mesothelioma have been constructed.
The combination of cisplatin and either pemetrexed or raltitrexed was associated with better quality of life and symptom control than cisplatin alone, with improvements in pain, dyspnea, fatigue, and cough, as well as global quality of life. The combination of cisplatin and raltitrexed improved dyspnea to a clinically meaningful extent compared with cisplatin alone, although other parameters remained stable. However, selection of patients who are fit for treatment is important, as is supportive care and careful oversight of potential toxicities. Treatment with cisplatin and pemetrexed should be accompanied by supplementary folate and vitamin B12 starting 1 to 2 weeks before the first day of chemotherapy.
Recently, the addition of bevacizumab to cisplatin and pemetrexed has demonstrated further benefits in progression-free and overall survival. Bevacizumab was used in combination with cisplatin and pemetrexed, and with subsequent single agent bevacizumab continuing until disease progression after completion of up to 6 cycles of the combination therapy. This addition gave a hazard ratio for overall survival of 0.7, increasing the median survival from 16.1 to 18.8 months, with manageable toxicities. The high cost of bevacizumab and modest benefits, as well as the lack of any predictive biomarker, has delayed widespread uptake into international routine clinical practice. However in selected patients without contraindications to anti-VEGF therapy this is an appropriate addition to standard chemotherapy.
Response to first-line chemotherapy should be assessed with serial CT performed at baseline and then after every two or three cycles. FDG-PET to monitor response is not standard, but can supplement information from CT and may provide an indication of response or progression at an earlier time point than CT. Although changes in serum mesothelin levels may parallel tumor volume, the test has not been sufficiently validated in this context to replace or supplement imaging to monitor response. The optimum number of cycles of platinum-based chemotherapy has not been demonstrated in clinical trials; however, four to six cycles of doublet chemotherapy is in keeping with the pivotal clinical trial data, allowing for cessation at four cycles if treatment is poorly tolerated. The spectrum of common cumulative toxicities includes fatigue, progressive anemia, and sensory peripheral neuropathy.
Timing of First-Line Chemotherapy
The timing of initiation of first-line palliative chemotherapy remains a matter of debate. A chemotherapy regimen used in one small randomized clinical trial was subsequently found to be inactive (mitomycin, vinblastine, and cisplatin or carboplatin) in a large, randomized study. In this trial, patients who were symptomatically stable were randomly assigned to receive either immediate chemotherapy or delayed treatment after the onset of symptoms. The investigators found a nonsignificant survival benefit for the immediate treatment arm. Nevertheless, in asymptomatic patients with minimal or nonmeasurable disease and epithelioid histology, for example, patients presenting with pleural effusion that has been effectively managed, it is reasonable to defer treatment if surgery is not planned.
Second-Line and Subsequent Chemotherapy
Patients often have a good performance status at progression after first-line chemotherapy and are fit for and desire further treatment. Although uncontrolled studies and anecdotal experience support the potential for second-line chemotherapy to elicit objective treatment responses, at the time of publication, no positive randomized controlled trial of any agent in the setting of uniform previous treatment with cisplatin and pemetrexed had been reported.
In the era before routine first-line treatment with a platinum agent and pemetrexed, authors of a well-conducted randomized clinical trial of second-line single-agent pemetrexed plus best supportive care compared with best supportive care alone in 243 patients reported partial response in 19% of patients receiving pemetrexed and 2% of patients receiving best supportive care alone. The disease control rate was 59% in the pemetrexed arm and 19% in the best supportive care arm, and progression-free survival favored the pemetrexed arm (median, 3.6 vs. 1.5 months) although there was no significant difference in overall survival ( p = 0.7434). Patients who had a response to their previous chemotherapy regimen were more likely to have a clinical benefit. Substantially more participants in the best supportive care arm (52% vs. 28%) received postdiscontinuation chemotherapy, which may have obscured any potential difference in overall survival.
Although these results are encouraging, this study does not give firm guidance in the post-pemetrexed setting. To attempt to address this question, patients from the pivotal trial of pemetrexed and cisplatin were evaluated for chemotherapy use after pemetrexed. Eighty-four patients in the pemetrexed and cisplatin arm received subsequent chemotherapy, with 48 patients receiving single-agent treatment and 36 patients receiving combination chemotherapy. Regimens included gemcitabine, vinorelbine, anthracyclines, and platinum agents alone and gemcitabine-based combinations. Although the data available were not suitable to examine response or disease control rates, subsequent chemotherapy was significantly correlated with longer survival ( p < 0.001), after adjustment for treatment group and prognostic factors. This finding may be explained by a benefit from second-line chemotherapy, but is also likely to be biased by the selection of fitter patients for subsequent treatment lines.
Support for reintroduction of pemetrexed-based chemotherapy for patients who had a previous response can be found in a number of uncontrolled clinical trials and case series ( Table 53.2 ). In an observational study, patients who had response or stable disease to previous pemetrexed-based therapy were further treated with pemetrexed either as a single agent (15 patients) or in combination with carboplatin or cisplatin (16 patients). The overall response rate was 19%, and 52% of patients had progressive disease as their best response. The median progression-free survival was 3.8 months, median overall survival was 10.5 months, and the toxicity was manageable. Predictors of better outcomes included a longer interval between initial pemetrexed-based chemotherapy and additional treatment, previous objective response to therapy, and second-line rather than third-line treatment.
| First-Line Treatment | Subsequent Treatment | No. of Patients | Objective Response Rate (%) | Disease Control Rate (%) | Progression-Free Survival (Mo) | Overall Survival (Mo) | Reference |
|---|---|---|---|---|---|---|---|
| Pemetrexed with or without platinum agent | Pemetrexed with or without platinum agent | 31 | 19 | 48 | 3.8 | 10.5 | Ceresoli et al. |
| Pemetrexed with or without platinum agent | Gemcitabine and vinorelbine | 30 | 10 | 43 | 2.8 | 10.9 | Zucali et al. a |
| Pemetrexed and platinum agent | Pemetrexed with or without platinum agent | 30 | 17 | 66 | 5.1 | 13.6 | Bearz et al. |
| None or 1 regimen | Gemcitabine and epirubicin | 23 | 13 (high dose), 7 (low dose) | NR | 4.2 | 5.7 | Okuno et al. |
| Various | Vinorelbine | 63 | 16 | 68 | NR | 9.6 | Stebbing et al. |
| Pemetrexed and carboplatin | Gemcitabine and docetaxel plus G-CSF | 37 | 19 | 62 | 7 | 16.2 | Tourkantonis et al. |
| Various (not pemetrexed) | Pemetrexed + BSC (vs. BSC) | 123 | 19 | 59 | 3.6 | 8.4 | Jassem et al. |
| Various | Irinotecan, cisplatin, and mitomycin C | 13 | 20 | 70 | 7.3 | 7.3 | Fennell et al. b |
| Platinum and pemetrexed | BNC105P | 30 | 3 | 46 | 1.5 | 8.2 | Nowak et al. c |
| Platinum and pemetrexed | Sunitinib | 53 | 12 | 77 | 3.5 | 6.1 | Nowak et al. d |
| Various | Pembrolizumab | 25 | 28 | 76 | 5.8 | NR | Alley et al. e |
| Platinum and pemetrexed | Avelumab | 53 | 9 | 58 | 4 | NR | Hassan et al. |
| Platinum and pemetrexed | Nivolumab | 18 | 27 | 50 | NR | NR | Quispel-Janssen et al. f |
a Zucali PA, Ceresoli GL, Garassino I, et al. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma . Cancer . 2008;112(7):1555–1561.
b Fennell DA, Steele JP, Shamash J, et al. Efficacy and safety of first- or second-line irinotecan, cisplatin, and mitomycin in mesothelioma. Cancer . 2007;109(1):93–99.
c Nowak AK, Brown C, Millward MJ, et al. A phase II clinical trial of the vascular disrupting agent BNC105P as second line chemotherapy for advanced malignant pleural mesothelioma. Lung Cancer . 2013;81(3):422–427.
d Nowak AK, Millward MJ, Creaney J, et al. A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol ., 2012;7(9):1449–1456.
e Alley EW, Schellens JH, Santoro A, et al. Single-agent pembrolizumab for patients with malignant pleural mesothelioma (MPM). Denver: World Conference on Lung Cancer: 2015.
f Quispel-Janssen J, et al. Nivolumab in malignant pleural mesothelioma (NIVOMES): an interim analysis, in 3th International Conference of the International Mesothelioma Interest Group. Birmingham, UK: 2016.
In a large retrospective assessment of patient outcomes with second-line therapy, 181 patients who had received second-line chemotherapy were identified from eight Italian centers. Most (66%) of the patients had received prior pemetrexed-based treatment, of whom 42 patients received further pemetrexed-based therapy and 31 received platinum and pemetrexed. Again, good performance status and more than 12 months since first-line therapy predicted better outcomes after further treatment in all patients. Although the disease control rate was similar for patients who were retreated with pemetrexed alone or in combination with a platinum drug, progression-free survival and overall survival were better for patients retreated with a combination of a platinum agent and pemetrexed. Clear potential biases exist that limit interpretation of these data, in particular, that selection of single-agent chemotherapy over combination chemotherapy is likely to have been influenced by clinician perception of the tolerability of combination chemotherapy for individuals, with single-agent treatment more likely to have been recommended for patients who were less fit.
A number of other nonpemetrexed second-line regimens were used in this retrospective Italian series, either with or without platinum agents. These regimens included combinations of cisplatin or carboplatin with gemcitabine or vinorelbine, as well as vinorelbine and gemcitabine alone or in combination. Overall, the disease control rate, progression-free survival, and overall survival were superior for patients who received repeat platinum-based treatment, however, we should interpret these data with the same potential for bias as just discussed.
The interpretation of these studies has substantial limitations, particularly in view of the heterogeneity of patient populations. In particular, in some studies not all patients had been previously treated with pemetrexed. Selection criteria and tumor measurement metrics were variable. Few studies included evaluation of symptom benefit, quality of life, or functional benefit as measured by lung function.
In recommendations for second-line therapy, it is appropriate to consider reintroduction of a pemetrexed-based regimen for patients who have had a response to first-line pemetrexed-based treatment, and who have had a long interval since previous treatment, with patients treated more than 12 months before reintroduction likely to have the most benefit. An appropriate regimen choice is pemetrexed with the alternative platinum drug or single-agent pemetrexed if the patient is unfit for combination therapy or has a contraindication to the platinum compounds. In patients for whom pemetrexed-based treatment is not recommended, such as patients with a shorter treatment-free interval, vinorelbine, either as a single agent or with gemcitabine, has an acceptable activity and toxicity profile. Treatment discussions should include careful consideration of patient preferences and disclosure regarding the limited data on functional and quality-of-life benefits.
A relative paucity of trials of second-line cytotoxic chemotherapy in malignant mesothelioma has been reported since 2009. Research efforts and enrollment into second-line trials have focused on identifying novel targeted agents that may have activity in mesothelioma and the prospect of molecular predictors of response to such agents. A realistic perception exists that the benefits of cytotoxic chemotherapy have reached a plateau, given the lack of development of new cytotoxic agents in favor of molecularly targeted agents, and there is likely to be limited benefit from further testing of existing cytotoxic agents in this setting. The current generation of clinical trials for second-line therapy in mesothelioma generates debate about an appropriate control arm. Placebo control is still considered appropriate for a phase III study, in the absence of data showing a survival benefit for second-line treatment after pemetrexed. Second-line trials should include testing of quality-of-life and functional parameters in order to draw robust conclusions about patient benefit in this population.
Surgery
Surgery for MPM is indicated for diagnosis, staging of disease, palliation of symptoms (rarely), and cytoreduction.
Palliative Surgery
Patients with mesothelioma most commonly present with dyspnea, chest wall pain, cough, or constitutional symptoms such as fatigue, fever, and anorexia. Respiratory symptoms may be secondary to atelectasis, with ventilation reduction and shunting caused by the compressive effects of pleural effusion or tumor encasement. Symptoms may also be secondary to altered respiratory mechanics as a result of altered chest wall mechanics and impaired movement of the ribs and diaphragm. Surgical palliation for patients with these symptoms includes treatment of pleural effusion and lung collapse and amelioration of chest wall mechanics.
Pleural Drainage
Treatment of pleural effusion depends on the size of the effusion, the degree to which it is causing atelectasis, and the amount of underlying lung entrapment. Simple thoracentesis is rarely effective in providing long-term relief of pleural effusion; however, it is a reasonable initial procedure to help dyspnea, to obtain a diagnosis, and to evaluate the degree to which the lung will reexpand. In the absence of complete reexpansion, pleural symphysis is unlikely to occur with sclerotherapy (i.e., talc). In cases of effusion with lung entrapment, placement of an indwelling pleural catheter can be helpful. This simple outpatient procedure does not require complete lung reexpansion to be effective, and, although tumor progression along the catheter tract has been described, it appears to be an uncommon event.
Palliative Pleurectomy
The aim of palliative pleurectomy is to free up an entrapped lung, allowing it to reexpand, to ameliorate the restrictive effect of chest wall tumor, and to reestablish pleural apposition and ultimately pleural symphysis. Palliative pleurectomy should be differentiated from cytoreductive PD, which is performed with the aim of achieving macroscopic complete resection of tumor in the hope of influencing time to recurrence and survival. Quality-of-life improvements after palliative pleurectomy have not been extensively documented, and no prospective comparisons between best supportive care and PD exist. Control of pleural effusion is good, with most reports citing success in 80% to 100% of cases. Improvement in dyspnea and chest wall pain is generally less. Soysal et al. retrospectively reviewed 100 consecutive pleurectomies performed for palliation of MPM. Pleural effusion was controlled in 52 (96%) of 54 patients who presented with symptomatic effusion, chest pain was relieved or improved in 85%, and cough and dyspnea improved in all patients. Most important, symptom relief was achieved for up to 6 months.
Although palliative pleurectomy can achieve excellent control of pleural effusion, it requires a thoracotomy, and the upfront morbidity of this procedure may negate some of its potential advantages, particularly with respect to pain control. VATS debulking has emerged as an alternative option. Waller et al. first described use of VATS pleurectomy in 19 patients with malignant effusion and showed control of pleural effusion in 84% of patients at a median follow-up of 12 months; however, tumor seeding at port sites occurred in 5 (38%) of 13 patients with MPM. A phase III trial comparing VATS pleurectomy with talc pleurodesis (MESOVATS) indicated that the success of VATS pleurodesis was higher than that of talc pleurodesis, but no differences in overall survival were found. The influence of histology on outcome following palliative pleurectomy was evaluated in 51 patients with MPM. Significant improvement in dyspnea and pain score was achieved at 6 weeks and 3 months; however, prolonged benefit was found mainly for patients with epithelioid tumors and for patients without substantial weight loss. The median survival for patients with nonepithelioid tumors in this study was 4.4 months. The 30-day mortality was 8%, but increased to 14% by 6 weeks, calling into question the validity of palliative pleurectomy for patients with sarcomatoid and biphasic tumors.
Cytoreductive Surgery
The aim of cytoreductive surgery is to remove all macroscopic tumor from the hemithorax. It is postulated, although unproven, that R0 or R1 cytoreduction prolongs survival for patients, particularly patients with epithelioid tumors and no lymph node metastases. The argument in favor of cytoreduction is supported by several observations. First, a large body of evidence from other disease sites shows a benefit to cytoreductive surgery in stage IV disease; the results of randomized trials support this procedure for advanced ovarian, colorectal, and renal cell cancers. Second, most long-term survivors of MPM have had surgery as a component of their therapy, whereas very few long-term survivors have been treated with nonoperative strategies. In two studies, both published in 2010, the outcomes for patients with MPM in the Surveillance Epidemiology and End Results database were evaluated. In both studies, survival was longer for patients who had cancer-directed surgery compared with patients whose treatment did not include surgery (11 vs. 7 months). Third, the median survival of patients in three phase III trials of chemotherapy was between 10 and 13 months, whereas in three multicenter trimodality phase II studies that included cytoreductive surgery in the form of EPP, the median survival on an intent-to-treat basis was notably longer than 17 months. Lastly, several retrospective studies appear to demonstrate longer survival for patients with mesothelioma who have undergone more complete cytoreduction compared with patients with larger tumor volumes after resection. The two approaches to cytoreductive surgery for pleural mesothelioma are EPP and radical PD (or total pleurectomy).
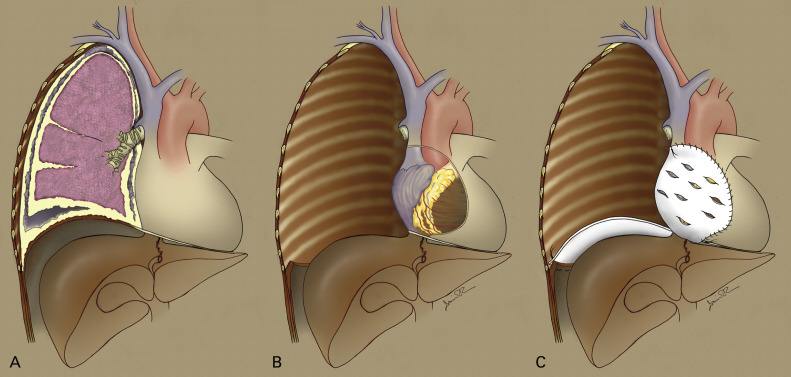
Extrapleural Pneumonectomy
EPP involves the en bloc resection of the parietal and visceral pleura, lung, ipsilateral pericardium, and diaphragm ( Fig. 53.2 ). The pericardium and diaphragm are usually reconstructed with prosthetic mesh, often polytetrafluoroethylene, although use of polyglycolic acid, polypropylene, and various biologic meshes has also been described. The procedure is extensive and has been associated with a high mortality rate, but in most large-volume centers perioperative mortality is below 8%. Postoperative morbidity is approximately 50% to 60% and most commonly includes supraventricular tachyarrhythmias (44% to 46%), which are easily treated, but also life-threatening events such as major hemorrhage (1%), cardiac herniation (1% to 2%), esophageal perforation (1% to 2%), bronchopleural fistula (1% to 2%), empyema (3% to 6%), acute respiratory distress syndrome (4% to 8%), and pneumonia (5% to 10%). Survival following EPP in the trimodality setting ranges from 10 to 28 months and is dependent on stage, histology, and whether survival is calculated from the date of surgery or from the date of diagnosis. Because the entire lung is removed with EPP, the at-risk area for local recurrence is limited to the inner aspect of the chest wall, the peridiaphragmatic area, and the ipsilateral mediastinum. Local recurrence rates with EPP alone range from 30% to 50%. For this reason, both adjuvant hemithoracic radiotherapy and intrapleural therapies, such as heated intrapleural chemotherapy (HIOC) and photodynamic therapy (described later), have been administered in an effort to reduce local recurrence.