Introduction
Overall, cancers of the endocrine system comprise 3.1% of the 1,806,590 new cancer cases estimated for 2020 . The majority of cases (95%) are attributed to thyroid cancer, which is the fifth leading site of new cancer cases in women ( Table 12.1 ). The majority of the nonthyroid cancers are rare, defined as fewer than 6 cases per 100,000 people per year . In contrast, endocrine-related malignancies in which exogenous hormones constitute risk factors for disease or in which malignancies are responsive to hormonal treatment comprise a much greater percentage of cancer cases. For example, breast and prostate cancers lead all other cancers in estimated new cases ( Table 12.1 ). This chapter on malignancy-associated endocrine disorders will examine the laboratory tests, as well as the epidemiology, risk and hereditary factors, pathology, clinical features, and additional diagnostic tests for cancers of the endocrine system.
| Total | Male | Female | ||||
|---|---|---|---|---|---|---|
| Site | # Cases | % All cases | # Cases | % All cases | # Cases | % All cases |
| Endocrine system | 55,670 | 3.1 | 14,160 | 1.6 | 41,510 | 4.5 |
| Thyroid | 52,890 | 2.9 | 12,720 | 1.4 | 40,170 | 4.4 |
| Other endocrine | 2780 | 0.2 | 1440 | 0.2 | 1340 | 0.1 |
| Endocrine related | ||||||
| Breast | 279,100 | 15.4 | 2620 | 0.3 | 276,480 | 30.3 |
| Ovary | 21,750 | 1.2 | 21,750 | 2.4 | ||
| Prostate | 191,930 | 10.6 | 191,930 | 21.5 | ||
| Testis | 9610 | 0.5 | 9610 | 1.1 | ||
Endocrine tumors can be potentially difficult to identify and may be complex to treat. Malignancies associated with some endocrine organs, such as the thyroid, do not produce clinical endocrinopathies, whereas malignancies associated with other organs may be diagnosed by measuring serum and urine concentrations of hormones if the tumor is functional. Overall, biochemical tumor markers are of value in most of the endocrine or endocrine-related malignancies with respect to diagnosis of disease, progression of disease, and monitoring the effects of therapy. Markers for malignancy include enzymes, hormones, oncofetal antigens, carbohydrate markers, proteins, oncogene products, genetic changes, and others . The serum and urine biochemical markers and genetic tests associated with the endocrine malignancies described in this chapter encompass many of these categories.
Multiple endocrine neoplasia syndromes
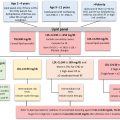
The multiple endocrine neoplasia (MEN) syndromes are disorders involving two or more endocrine glands typically inherited in an autosomal dominant fashion, although sporadic cases may occur, which are manifested by tumors, both benign and malignant. Tumors may be functional or nonfunctional. Based on the combinations of endocrine tumors involved, MEN syndromes have been classified as: (1) MEN1 (Werner syndrome) characterized by menin mutations, (2) MEN2A (Sipple syndrome) and (3) MEN2B (previously MEN3) characterized by RET proto-oncogene mutations, and (4) MEN4 characterized by CDNK1B mutations. Germline mutations in these disorders in conjunction with biochemical measurements and clinical observations aid in the early diagnosis and management of the diseases.
MEN1
Epidemiology
MEN1 is a very rare disease that occurs in 1 out of 30,000 individuals. Clinical manifestations usually develop in the third or fourth decade.
Risk and inheritance factors
The gene for MEN1, located on chromosome 11q13, was cloned in 1997. The gene encodes a 610-amino acid nuclear protein, menin, a scaffolding protein that regulates gene transcription; however, its function in tumorigenesis is unknown. MEN1 has an autosomal dominant inheritance pattern. The gene appears to be a tumor suppressor gene in which tumorigenesis results from the loss of heterozygosity. In addition to germline mutations, somatic mutations have been found in sporadic tumors. Both sexes are affected equally.
Characterization
Specific characteristics associated with MEN1 are listed in Table 12.2 . The parathyroid, pancreatic islet cells, and the anterior pituitary (three “Ps”) are primarily affected, although the presentation of the disease varies among individuals, contributing to underdiagnosis of the disease. A diagnosis of MEN1 has been defined as (1) clinical: an individual with two or more endocrine tumors, (2) familiar: an individual with one MEN1-associated tumors with a first-degree relative with a clinical diagnosis, and (3) genetic: an individual with a germline mutation that does not yet have clinical or biochemical manifestations. The majority of tumors are benign, with malignant tumors limited to the pancreatic islets. The parathyroid gland is involved in >95% of cases. Hyperparathyroidism, resulting from multiglandular nodular hyperplasia, is often one of the first indicators of this disease. Hyperparathyroidism is commonly discovered in the second decade of life when screening family members of affected MEN1 individuals. In adults, hyperparathyroidism is often discovered biochemically when MEN is suspected or upon additional testing when pituitary or pancreatic disease is found.
| MEN1 |
|
| MEN2 |
|
| MEN4 |
|
Pancreatic neuroendocrine tumors (PNETs) (pancreatic islet cell tumors) are a manifestation of MEN1 in 30%–80% of cases. The most common tumors are gastrinomas (60%), characterized as small, multiple adenomas located in the pancreas or duodenum. Most gastrinomas associated with MEN1 are malignant. Other pancreatic tumors found in MEN1 include: insulinomas, pancreatic polypeptidomas (PPomas), and more rarely vasoactive intestinal peptidomas (VIPomas), and glucagonomas. More than one type of tumor may be present in the same patient. Nonfunctioning NETs are increasingly identified from radiological studies and have a worse prognosis than functioning tumors. Chromogranin A, neuron-specific enolase (NSE), pancreatic polypeptide, and others may be secreted by nonfunctioning tumors, but are not associated with a specific clinical syndrome.
Anterior pituitary adenomas, another feature of MEN1, are present in 15%–50% of cases. Most pituitary adenomas are functional and commonly secrete prolactin. Growth hormone and adrenocorticotropic hormone (ACTH) are secreted less frequently, and elevated concentrations result in acromegaly or Cushing disease. Tumors may also be nonfunctional. In addition to the parathyroid, pancreatic, and pituitary involvement in MEN1, patients have an increased frequency of adrenal cortical hyperplasia or adenomas, carcinoid tumors in the thymus (men primarily) and bronchus (women primarily), lipomas, and thyroid adenomas.
Diagnostic and screening laboratory tests
Patients diagnosed with hyperparathyroidism (multiglandular disease or hyperplasia), Zollinger–Ellison syndrome (ZES), or insulinoma may be at increased risk for MEN1 and thus should be questioned regarding a family history of MEN1 and potential screening initiated. Over 1000 mutations in the MEN1 gene have been identified, although there is no correlation between genotype and phenotype. Patients with MEN1 are not treated until there is evidence of disease and are not treated prophylactically; however, patients who are likely carriers of the MEN1 mutation should be followed closely. Direct DNA testing is used to detect MEN1 germline mutations and is recommended for carrier identification. Candidates include index cases to confirm a clinical diagnosis, first-degree relatives of mutation carriers to identify those who require or do not require screening, and some cases with features that are suspicious or atypical for MEN1. All MEN1 kindreds are likely to have an MEN1 gene mutation, although testing may not detect 10%–20% of mutations.
In the absence of or in conjunction with genetic testing, diagnoses can be made on the basis of clinical presentation and elevated laboratory values. Similar to sporadic disease, history and physical exams should focus on signs and symptoms of hypercalcemia, nephrolithiasis, peptic ulcer disease, hypoglycemia, galactorrhea-amenorrhea, Cushing disease, hypopituitarism, and acromegaly. Similar to sporadic disease, MEN1 patients with primary hyperparathyroidism are often asymptomatic and diagnosis is based on elevated serum calcium and parathyroid hormone (PTH) concentrations. In contrast to hyperparathyroidism in sporadic disease, age of onset is earlier (MEN1: 20–25 years and sporadic disease: 55–60 years), male to female ratio is equal compared to greater preponderance in women in sporadic disease, and all four parathyroid glands are commonly affected compared to primarily a single adenoma in sporadic disease. In PNET, the majority of tumors (>50%) secrete gastrin with other polypeptides secreted including insulin, glucagon, and vasoactive intestinal polypeptide (VIP). Anterior pituitary tumors secrete excess prolactin (60%), producing amenorrhea, infertility, and galactorrhea in women and impotence in men, growth hormone (25%) producing acromegaly, or ACTH (6%) producing Cushing disease. Guidelines for periodic screening in index cases and those at high risk for MEN1 have been developed with biochemical screening yearly and baseline imaging of the pituitary, pancreas, and adrenals repeated every 1–3 years and thymic and bronchial carcinoids every 1–2 years. Laboratory testing of serum calcium, PTH, gastrin, fasting glucose and insulin, VIP, pancreatic polypeptide, chromogranin A, prolactin, and insulin-like growth factor 1 (IGF-1) is suggested overall with specific testing in those in which a particular syndrome is suspected.
MEN2
Classification
MEN2 is classified into two syndromes: MEN2A (95% of cases) and MEN2B. MEN2A is further classified into four variants: (1) classical MEN2A, which is the most common variant; (2) MEN2A with cutaneous lichen amyloidosis (CLA); (3) MEN2A with Hirschsprung disease (HSCR); and (4) familial medullary thyroid cancer (FMTC). Rearranged during transfection ( RET ) germline mutations are present in families or individuals with FMTC and have MTC like the other variants, but neither pheochromocytomas nor hyperparathyroidism, while either or both may manifest in the other variants depending upon genotype.
Epidemiology
MEN2A and MEN2B are rare diseases with an estimated incidence of 1 in 80,000–200,000 for MEN2A and 1.4–2.6 per million live births a year for MEN2B. The 10-year survival is MEN2A is 97.4% compared to 75.5% in MEN2B.
Risk and inheritance factors
MEN2A (80% cases), MEN2B (5% cases), and FMTC, a variant of MEN2A (15% cases), are autosomal dominant syndromes resulting from mutations of the RET proto-oncogene on chromosome 10 (10q11.2). The RET proto-oncogene is a receptor tyrosine kinase. Receptor tyrosine kinases are found on cell surfaces and transduce signals for cell growth and differentiation. RET is expressed in the C-cells of the thyroid gland and in other tissues including the adrenal medulla and neurons. The mutations are activating gain-of-function, unlike other cancer syndromes, which involve inactivation of tumor suppressor genes or DNA mismatch repair genes. In MEN2A, point mutations are primarily found in codons in specific cysteine residues in the RET extracellular domain (primarily exons 10 and 11) and less commonly the intracellular tyrosine kinase domains (e.g., exons 13–15 associated with FMTC). Ninety-eight percent of MEN2A and FMTC have an identifiable RET mutation. In MEN2B there is a point mutation in the intracellular TK2 domain at codon 918 in exon 16 in 95% of cases. RET mutations have also been found in sporadic disease (MTC and pheochromocytoma). Men and women are equally affected and penetrance is age-related.
Characterization
Specific characteristics associated with MEN2 are listed in Table 12.2 . MEN2A and MEN2B are similar in some respects, yet also have distinct characteristics. In both syndromes, virtually all patients develop MTC and depending upon genotype, approximately 20%–50% of MEN2A and 50% of MEN2B develop pheochromocytomas. Thyroid C-cell and adrenal chromaffin cell hyperplasias precede the development of multifocal clonal tumors. In contrast, 20%–30% of MEN2A patients develop hyperparathyroidism. Parathyroid involvement is rare in MEN2B. MEN2B patients, however, have a distinctive physical appearance not found in MEN2A. Neural defects include mucosal neuromas of the lips, eyelids, and tongue. An additional distinctive physical feature is a marfanoid habitus with excessive limb length, hyperflexible joints, scoliosis, and anterior chest wall deformities. Neural abnormalities may be present in the gastrointestinal (GI) tract with the presence of ganglioneuromatosis. This may affect motility of the gut resulting in diarrhea, constipation, or a clinical picture of megacolon. Ocular manifestations include alacrima, mild ptosis, eversion of upper eyelids, conjunctival neuromas, and prominent corneal nerves.
MTC differs in peak incidence between MEN2A and MEN2B with peaks in the second and third decades for MEN2A and peaks in the first and second decades for MEN2B. The MEN2B phenotype is more aggressive, which may be related to stage at diagnosis. In contrast to sporadic MTC tumors that are single and unilateral, familial forms are multifocal and bilateral. A thyroid nodule, goiter, mass, or cervical lymphadenopathy is present in a third of MEN2 patients. Cushing syndrome, from ACTH secretion, and diarrhea or flushing, manifestations of excessive hormone production, or tumor secretions, may be present. The majority of MTC cases are detected in asymptomatic high-risk relatives through screening procedures. In FMTC, MTC only is present with no evidence of pheochromocytoma or hyperparathyroidism. The disease has a late presentation with a course less aggressive than MEN2A and MEN2B.
Pheochromocytomas appear after thyroid disease in 50%, concurrently in 40%, and prior in 10% of patients with presentation in the fourth and fifth decades. Malignant tumors are rare. Severe hypertension, stroke, myocardial infarction, or death may result if untreated. In familial disease sustained hypertension is unusual with early symptoms of anxiety, palpitations, and tachycardia, and later symptoms of headache, pallor, and perspiration.
Hyperparathyroidism in MEN2A is typically diagnosed at the same time as MTC and typically multiglandular in nature. Hypercalcemia is typically mild and 85% of patients are asymptomatic.
Diagnostic and screening laboratory tests
Patients with MTC or pheochromocytoma should be investigated for the presence of MEN2 syndromes since MEN is often discovered in a family following diagnosis of either of these conditions. Forty percent of MEN2A carriers do not have symptoms; thus a negative family history does not rule out the presence of the syndrome. Clinical evaluation of patients with suspected or known MEN2 or MEN2B and at-risk relatives includes biochemical testing, imaging to localize associated tumors, and genetic counseling and testing. Germline RET testing is indicated in patients with a clinical diagnosis of MEN2A (MTC and pheochromocytoma), clinical characteristics of MEN2A variants including CLA and HSCR, MEN2B phenotypic manifestations, and sporadic MTC, C-cell hyperplasia, or unilateral or bilateral pheochromocytoma. Initial testing should include DNA sequencing of exons 10, 11, and 13–16 or the entire RET coding region. More limited testing may be done when specific clinical phenotypes are present, for example, in MEN2B where the exon 16 (codon M918T) mutation may be tested first. Testing at-risk family members where the germline RET mutation is known facilitates a targeted approach focusing on the specific codon housing the mutation. Early identification of RET germline mutations is critical to avoid associated morbidity and mortality allowing treatment and monitoring in those asymptomatic. Prophylactic thyroidectomy can prevent or potentially cure MTC. The American Thyroid Association (ATA) and other groups have established risk categories for patient management of both children and adult RET mutation carriers. Timing of thyroidectomy is based on the specific mutation and its clinical behavior. In the highest and high-risk categories, which include MEN2B mutations, surgery is recommended before ages 1 and 5 years, respectively, while serial serum calcitonin measurements and neck ultrasounds are incorporated for those at moderate risk. Timing and frequency of screening procedures for pheochromocytoma and hyperparathyroidism are similarly recommended by risk group. In adults with RET mutations the initial laboratory assessment is performed with serum calcitonin and carcinoembryonic antigen (CEA), and plasma-free metanephrines or urinary fractionated metanephrines with annual monitoring if calcitonin concentrations are normal. If elevated, further evaluation is performed for staging of MTC and/or pheochromocytoma in advance of treatment. Guidelines for managing patients following thyroidectomy for monitoring disease status include serum calcitonin measurements at defined intervals with concentrations >150 pg/mL triggering imaging for detecting metastases and concentrations that are detectable, yet <150 pg/mL with no evidence of disease triggering frequent measurements every 3–6 months to determine doubling times for calcitonin and CEA for assessing rate of MTC progression. Periodic surveillance for MEN2A and MEN2B-associated tumors is recommended for patients with a clinical diagnosis or suspicion of MEN, even if RET testing did not identify a mutation, and in at-risk relatives with negative or no genetic testing performed.
MEN4
Although the majority of patients with MEN1 have mutations in the MEN1 gene, approximately 5%–10% may have other mutations. MEN4 is a MEN1-like syndrome in patients with mutations in the CDNK1B gene that encodes a 196-amino acid cyclin-dependent kinase inhibitor (CK1) p27 kip1 , which is a cell cycle regulating protein. CDNK1B is also a tumor suppressor gene and eight heterozygous loss-of-function mutations have been identified. Patients with MEN4 primarily have parathyroid tumors (80% cases), pituitary tumors (40% cases), and duodenopancreatic NETs (35% cases) and gonadal, adrenal, renal, and thyroid tumors. Investigation and treatment of tumors in MEN4 are similar to MEN1 and non-MEN1 tumors.
Gastrointestinal neuroendocrine tumors
Carcinoid tumors
A description of carcinoid tumors is found in Chapter 6 : Gastroenteropancreatic Tumors.
Gastrinoma (Zollinger–Ellison syndrome)
Epidemiology
Gastrinomas are rare with an incidence of 1 per million. The majority are found in the duodenum, particularly in those with MEN 1 (70%–100%), with only 25% in the pancreas. ZES, defined by increased gastric acid production and peptic ulcerations, may account for 0.1% of patients with duodenal ulcer disease. Gastrinomas are the most common hormone-secreting malignant pancreatic tumors.
Risk and hereditary factors
Twenty-five to thirty percent of tumors are associated with the MEN1 syndrome.
Pathology
Sixty to ninety percent of tumors are malignant. Metastases are to lymph nodes, primarily, and to the liver. Duodenal gastrinomas are less likely to have metastasized to the liver at diagnosis. The time between symptom onset and diagnosis averages greater than 5 years and 25% of patients have metastatic disease at presentation.
Clinical features and laboratory tests
ZES symptoms are nonspecific and can be masked by use of proton pump inhibitors. Most common symptoms include abdominal pain, heartburn, diarrhea, and weight loss. Unexplained hypercalcemia, from MEN1 syndrome, may provide a clue in the ZES diagnosis. Laboratory testing associated with diagnosis of gastrinoma includes fasting serum gastrin measurements with concentrations >1000 pg/mL or >10 times the upper limit of normal (ULN) with corresponding gastric acid hypersecretion (gastric pH ≤2) diagnostic although gastrin concentrations are not commonly this high (<9% of patients). Proton pump inhibitors should be discontinued before gastrin testing. No further testing is needed if gastric pH is >2 or gastrin concentrations are normal and pH is ≤2. Patients with elevated serum gastrin (1–10× ULN) and gastric pH below 2 undergo gastrin provocation with secretin stimulation, which has supplanted measurement of basal acid output. Secretin is infused intravenously at 0.4 µg/kg over 1 min with gastrin measurements at baseline and 2, 5, and 10 min postinfusion. Cutoffs for a positive test include a 50% increase in gastrin concentration or increases ≥110 or 200 pg/mL. Although less specific than gastrin, chromogranin A can be elevated in gastrinoma and is used as an adjunctive diagnostic test. Chromogranin A can also be used as a tumor marker to monitor treatment effects. Following ZES diagnosis, a number of imaging modalities can be employed for tumor localization and assessment of tumor size and metastases including computed tomography (CT)/magnetic resonance imaging (MRI), endoscopic ultrasound, and somatostatin receptor-based imaging.
Glucagonoma
Epidemiology
Glucagonomas have an incidence of 1 in 20 million per year with a peak incidence between ages 40 and 60.
Risk and hereditary factors
Twenty to fifty percent of glucagonomas are associated with MEN1, although they only occur in 3% of patients with MEN1.
Pathology
Tumors, found in the islet alpha cells of the pancreas, are large and slow-growing. Seventy-five percent are malignant and 75% are metastatic at diagnosis. The tumors can result in elevated levels of peptides such as pancreatic polypeptide, gastrin, somatostatin, and insulin, in addition to glucagon.
Clinical features and laboratory tests
Fasting plasma glucagon concentrations greater than 1000 pg/mL are diagnostic. Hypercalcemia and hypoaminoacidemia (alanine, glycine, and serine concentrations <25% of normal) may also be present. Clinical features include skin rash (necrolytic migratory erythema), weight loss, glucose intolerance/diabetes mellitus, and anemia.
Insulinoma
Epidemiology
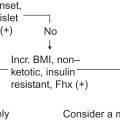
Insulinomas, the most common islet cell tumors of the endocrine pancreas, have an incidence of 4 per million per year with the largest incidence between ages 40 and 60 years with higher incidence in women compared to men. Incidence is earlier, before the age of 40, in patients with MEN1.
Risk and hereditary factors
The majority of tumors are sporadic while 10% occur in association with MEN1.
Pathology
Ninety-nine percent of tumors arise in the β cells of the pancreas and 80% are hormonally functional. Ten percent of tumors are malignant, although slow-growing. Fifty percent of patients with malignant insulinomas have metastases at diagnosis, primarily to the liver and regional lymph nodes.
Clinical features and laboratory tests
The diagnosis of hypoglycemia (Whipple’s triad) and the diagnostic workup for the causes of hypoglycemia are described in Chapter 7 , Evaluation of Hypoglycemia. The diagnosis of insulinoma is aided by measurements and observations made during a supervised prolonged fast (48 or 72 h). During the fast, patients with insulinoma will develop neuroglycopenic symptoms resulting from glucose concentrations <45 mg/dL. Concurrently, serum insulin concentrations will be increased and the insulin to glucose ratio will be >0.3. Similar to insulin, C-peptide concentrations will not be suppressed with hypoglycemia. Proinsulin concentrations greater than 22%–25% of plasma insulin immunoreactivity are also present in insulinoma.
Somatostatinoma
Epidemiology
Somatostatinomas are exceedingly rare, with an incidence of 1 in 40 million.
Risk and hereditary factors
Somatostatinomas occur in <1% of patients with MEN1 and can be associated without a functional clinical syndrome in up to 10% of patients with von Recklinghausen’s neurofibromatosis.
Pathology
Approximately 60% of tumors are located in the D-cells of the pancreas (50% head, 25% tail) with the majority of others located in the small intestine. Eighty percent of pancreatic and 50% of intestinal tumors are malignant. Most tumors are large and metastases are present in the majority of cases at diagnosis.
Clinical features and laboratory tests
Plasma somatostatin concentrations in somatostatinoma reach concentrations 50 times the upper limit of the reference range. However, the characteristic triad of the functional somatostatinoma syndrome, diabetes mellitus, cholelithiasis, and diarrhea, has not been reported in MEN1. More detail on somatostatinomas can be found in Chapter 6 , Gastroenteropancreatic Tumors.
VIPoma (vasoactive intestinal polypeptide)
Epidemiology
VIPoma, with an associated clinical syndrome referred to as pancreatic cholera syndrome, Verner–Morrison syndrome, or WDHA syndrome (watery diarrhea, hypokalemia, achlorhydria or hypochlorhydria), has an incidence of 1 in 10 million. The majority of VIPomas are sporadic with approximately 5% associated with MEN1.
Pathology
The majority (90%) of tumors in adults occur in the pancreatic body or tail with 10% in the colon, bronchus, adrenals, liver, and sympathetic ganglia. The adrenals and sympathetic ganglia are the most common tumor sites in children. Seventy to ninety percent of tumors are malignant.
Clinical features and laboratory tests
In addition to characteristic clinical features, serum VIP concentrations can aid in VIPoma diagnosis (values >200 pg/mL). Diarrhea (>700 mL/day) is secretory with a low osmotic gap. VIPoma is described in more detail in the chapter on gastroenteropancreatic tumors.
Adrenal tumors
Adrenocortical carcinoma
Epidemiology
Adrenocortical carcinoma (ACC) is rare with an incidence of two per million. Incidence peaks are in children less than 5 years of age and between ages 40 and 50. 1.3% of childhood cancers are adrenal carcinoma in contrast to 0.02%–0.2% of adult cancers. Two-thirds of occurrences are in women. Median overall survival is 3–4 years, although prognosis is heterogeneous.
Risk and hereditary factors
The majority of tumors are sporadic, although adrenal cancer occasionally occurs in hereditary syndromes including MEN1, Li-Fraumeni syndrome, Beckwith–Wiedemann syndrome, and Lynch syndrome. There are also reports of ACC in patients with familial adenomatous polyposis (FAP), neurofibromatosis type 1, and Carney complex. Increased incidence of ACC in children is attributed to germline TP53 mutations present in 50%–80% of patients. Increased incidence in children in Southern Brazil is ascribed to the high prevalence of the TP53 p.R337H mutation. Overall, mutations in the tumor suppressor gene TP53 are the most common somatic mutation present in a third of ACC.
Pathology
Fifty percent of tumors are functional, producing cortisol and other steroids. Tumors are highly aggressive. Twenty-five to thirty percent of patients have metastases at diagnosis, primarily to lymph nodes, lung, liver, and bone. Weiss criteria are used to histologically distinguish adrenocortical cancer from adrenocortical adenoma in addition to specific immunohistochemical studies.
Clinical features
In patients with nonfunctioning tumors, abdominal masses are often discovered in patients with abdominal pain and with symptoms of weight loss, fullness, indigestion, nausea, vomiting, pain, or weakness. Twenty to thirty percent of ACCs are incidentally found through imaging procedures. In patients with functioning tumors, symptoms of nonfunctioning tumors plus those due to excess hormone may be present (40%–60% of patients at presentation). Features associated with Cushing syndrome are described in Chapter 4 , Disorders of the Adrenal Gland. Virilization, or hirsutism in women, and feminization in men, may be present; hypercortisolism (Cushing syndrome) is present in 50%–70% of hormone-secreting ACC, androgen excess (virilization) in 20%–30% of female patients, estrogen excess (feminization) in 5% of male patients, and mineralocorticoid excess in 2%–3% of patients. In most cases (24%–35%) a number of steroid hormones are produced in contrast to an increased production or secretion of one adrenocortical steroid more indicative of a benign neoplasm. Androgen or estrogen production and symptoms of rapidly progressing Cushing syndrome are suggestive of malignancy.
Laboratory tests
A detailed description of the diagnosis of ACTH-independent Cushing syndrome can be found in Chapter 4 , Disorders of the Adrenal Gland. Malignant, but not benign, tumors may produce steroids other than cortisol due to partial enzyme deficiencies in steroid biosynthesis pathways. Therefore increased urinary 17-ketosteroid excretion and increased plasma DHEAS (dehyroepiandrosterone sulfate) concentrations are diagnostic of carcinoma. 17-Ketosteroids are also useful for following treatment. In women with symptoms of virilization, concentrations of plasma testosterone and androstenedione may be elevated, while in men with feminization plasma estrone and estradiol may be elevated. Hyperaldosteronism can be evaluated with increased aldosterone concentrations and suppressed plasma renin activity. Patients with adrenal masses should undergo laboratory assessment for pheochromocytoma (see the following section and Chapter 4 : Disorders of the Adrenal Gland).
Tumor imaging
Adrenal tumors are often incidental findings on radiology studies. Large tumors >4–6 cm or increasing tumor growth over a 6-month period are suggestive of carcinoma. Tumors are bilateral in 2%–10% of cases. Chest CT and CT or MRI of the abdomen/pelvis are used to image adrenal tumors to aid in diagnosis, staging, and follow-up. There is a lack of consensus in the use of fluorodeoxyglucose-positron emission spectroscopy (FDG-PET)/CT.
Neuroblastoma
Epidemiology
Neuroblastoma and ganglioneuroblastoma comprise 6% of childhood cancers (ages 0–14 years) and comprise the third most common malignancy in childhood, following acute lymphocytic leukemia, and brain and central nervous system (CNS) cancers. The incidence of neuroblastoma is 2–10 cases per million. It is the most common cancer in children under 5 years of age and 60% of diagnoses are before age 3 and 90% before age 5. The tumor rarely presents in older individuals. The 5-year survival rate is 80%, although is variable from spontaneous regression to widespread metastatic disease that does not respond to treatment. Prenatally diagnosed neuroblastoma has increased owing to ultrasound examination. Greater than 90% of congenital neuroblastomas arise from the adrenal gland. Most have favorable features and spontaneously regress during the first year of life.
Risk and inheritance factors
Neuroblastoma is more common in whites than in other races/ethnicities and slightly more common in boys than in girls. The majority of neuroblastomas are sporadic, only 1%–2% of cases are familial. Amplification of the MYCN oncogene is present in 20% of patients and is associated with aggressive disease and a poor prognosis. Activating mutations in ALK , PHOX2B , and KIF1B genes have also been identified in hereditary neuroblastoma.
Pathology
In neuroblastoma, 97% of neuroblastic tumors are embryonic tumors that are neural crest in origin and arise from sympathetic or adrenal neuroblasts. Thirty percent of neuroblastomas occur in the adrenal medulla, 60% in the abdominal paraspinal ganglia, and the remaining in the sympathetic ganglia in the chest, head/neck, and pelvis. Common metastatic sites include regional lymph nodes, liver, cortical bone, skin, and bone marrow. The paraneoplastic opsoclonus-myoclonus syndrome occurs in 2%–3% of patients.
Clinical features
Clinical symptoms depend on the anatomic location of the tumor. Adrenal tumors produce abdominal masses and may result in abdominal pain and distension. A palpable mass may be found on routine examination, while hypertension may result from renal vessel compression. Other nonspecific symptoms include: anorexia, fever, diarrhea, and bone pain. Dark blue skin lesions can also be observed. One in five patients is hypertensive; secretion of inactive catecholamine precursors as opposed to more active forms may be the explanation. Diagnosis is made by histological confirmation of an excisional biopsy or the primary tumor. Bilateral bone marrow biopsy biopsies/aspirates are performed for evaluating disease extent.
Laboratory tests
The majority of tumors metabolize catecholamines, which can be measured in urine and used for diagnosis as well as for monitoring treatment. Vanillylmandelic acid (VMA), and homovanillic acid (HVA) may have increased excretion. The ratio of urinary VMA to HVA <1.5 is indicative of a worse prognosis as are serum ferritin and LDH. NSE, a glycolytic enzyme found in tumors associated with neuroendocrine origin, is elevated in greater than 90% of children with advanced disease. Concentrations appear to correlate with the stage of the disease and higher concentrations are also indicative of a poorer prognosis. Use of NSE for monitoring is controversial.
Tumor imaging
Ultrasound is typically the first imaging modality for an abdominal mass followed by CT or MRI for tumor characterization and evaluation of image-defined risk factors that are part of staging criteria guidelines of the International Neuroblastoma Risk Group Staging System (INRGSS). Metaiodobenzylguanidine (MIBG) scintigraphy is part of the INRGSS for staging. MIBG is a compound that resembles norepinephrine and is taken up into neurosecretory storage vessels. Labeling with 123 I allows identification of primary and metastatic tumor sites following injection. MIBG scintigraphy is approximately 90% sensitive and 100% specific for neuroblastoma. MIBG scintigraphy can also determine eligibility for potential therapy with 131 I MIBG.
Pheochromocytoma
Epidemiology
Pheochromocytomas are very rare except when associated with familial disease. The incidence is 3–8 cases per million per year. It is estimated that the prevalence in patients with hypertension is 0.1%–0.6% of patients and 5% of patients have pheochromocytoma discovered incidentally on imaging studies. Diagnoses are most commonly made in the fourth and fifth decades and 10%–20% percent of patients are children.
Risk and hereditary factors
Pheochromocytomas are associated with hereditary syndromes in approximately 30%–40% of cases, while somatic mutations are present in 40%–50% of ~20 identified susceptibility genes (see Table 4.4 in Chapter 4 , Disorders of the Adrenal Gland). In patients with germline RET mutations such as in MEN2, tumors are almost entirely adrenal.
Pathology
Pheochromocytoma and paraganglioma are similar catecholamine-secreting tumors of chromaffin cells that differ in anatomic location: adrenal medulla and sympathetic paravertebral ganglia, respectively. Eighty to eighty-five percent are pheochromocytomas (10% in both glands).
In addition to secreting one or more of the catecholamines: epinephrine, norepinephrine, and occasionally dopamine, tumors may also secrete VIP, serotonin, calcitonin, ACTH, angiotensin-converting enzyme, renin, somatostatin, and neuropeptide Y. Benign and malignant pheochromocytomas are difficult to distinguish with similar histologic and biochemical features. Malignancy is defined as metastasis in nonchromaffin tissue. The WHO classification of endocrine tumors endorses the term metastatic pheochromocytoma, as all pheochromocytoma may have metastatic potential. The prevalence of metastatic disease is 10%–17%, but can be >40% in patients with germline mutations of the succinate dehydrogenase-B ( SDHB ) gene. Metastases are found in bone, liver, lung, and soft tissues. Metastatic spread is more common in extra-adrenal tumors.
Clinical features and laboratory tests
There is variability in the clinical presentation of pheochromocytoma depending upon specific catecholamine secretion, although the classic triad of presenting symptoms has included headaches, palpitations, and profuse sweating. Hypertensive episodes can result from catecholamines acting on adrenergic receptors. Initial biochemical testing per Endocrine Society guidelines includes plasma-free metanephrines (collected supine) or urinary fractionated metanephrines that have sensitivities and specificities of 97% and 80%–100%, and 97% and 91%, respectively. These metabolites are continuously produced by tumors, while catecholamine release can be low or episodic. Follow-up testing with the clonidine suppression test or the combination of urinary fractionated metanephrines with chromogranin A has been proposed when plasma-free metanephrine results are borderline and collection and other preanalytical considerations (e.g., supine position, drug interference) have been ruled out., A false-positive rate for metanephrine testing of 19%–21% has been reported. See Chapter 4 , Disorders of the Adrenal Gland, for a complete description of the clinical features and diagnostic laboratory tests for pheochromocytoma. An additional laboratory test to specifically aid in the pathologically difficult diagnosis of malignant tumors is the neuroendocrine serum tumor marker NSE. Benign tumors have not been shown to produce this marker.
Tumor imaging
Initial tumor imaging following biochemical evidence of pheochromocytoma is accomplished with CT scans or MRI with 18 F-FDG-PET/CT for patients with known metastatic disease and 123 I MIBI (metaiodobenzylguanidine) scintigraphy when 131 I MIBI radiotherapy is planned.
Parathyroid and thyroid tumors
Parathyroid
Epidemiology
Parathyroid carcinoma is very rare. Less than 1% of hyperparathyroidism is attributable to parathyroid malignancy. The mean age at diagnosis is 45 with equal distributions between men and women, whereas mean age at diagnosis is 10 years later with a greater preponderance in women in benign hyperparathyroidism. Racial differences have not been observed. Five- and ten-year survival are 85%–91% and 49%–88%, respectively.
Risk and hereditary factors
Parathyroid cancer is primarily sporadic, although it may be associated with familial syndromes such as hyperparathyroidism-jaw tumor syndrome (HPT-JT), MEN1, MEN2A, and familial isolated hyperparathyroidism (FIHP). Germline-inactivating mutations of CDC73 (formerly HRPT2 ) occur in HPT-JT, where ~15% of patients may have parathyroid cancer and FIHP. Somatic and germline mutations are present in 80% of sporadic parathyroid carcinoma cases. Germline mutations may suggest HPT-JT or a phenotypic variant. The CDC73 gene encodes the nuclear tumor suppressor protein parafibromin, a cotranscription factor involved in chromosome remodeling. There is increased incidence of parathyroid carcinoma in patients with prior neck irradiation and with end-stage kidney disease.
Pathology
Less than 0.5% of parathyroid tumors are malignant. Tumors have an indolent course owing to low malignant potential, although recurrence is common. At diagnosis lymph node involvement (<5%) and distant metastases (<2%), typically to the bone, liver, and lung, are uncommon. Morbidity and mortality are a result of hypercalcemia as opposed to spread of the tumor. Carcinoma during parathyroidectomy may be suspected when a large mass (3.0–3.5 cm) that adheres to and/or infiltrates surrounding tissues is present. Histological criteria for diagnosis include capsular, vascular, and/or perineural tumor invasion. Distinguishing parathyroid carcinoma from adenoma is difficult and diagnosis may be made after surgery and even after disease recurrence. Preoperative fine-needle aspiration is not recommended due to difficulty in differentiating benign and malignant disease and potential for tumor rupture and seeding.
Ninety to ninety-five percent of tumors are functional. Patients with nonfunctioning tumors have normal concentrations of serum calcium and PTH and have delayed diagnosis and a poorer prognosis, and in contrast to patients with functioning tumors, mortality is more commonly a result of tumor burden.
Clinical features
A palpable neck lesion, present in 30%–50% of patients, in the setting of hyperparathyroidism is suggestive of parathyroid cancer. Signs and symptoms are a result of primary hyperparathyroidism and resulting high serum calcium concentrations and are more severe than in benign disease. Bone effects (50% of patients) include: osteitis fibrosa cystica, osteopenia, pathologic fractures, and bone pain. Bone involvement is suggested when serum alkaline phosphatase is markedly high when liver disease is absent. Renal effects (60% of patients) include: nephrolithiasis, nephrocalcinosis, polyuria, polydipsia, and decreased renal function. Neuromuscular effects include: muscle weakness and aches, easy fatigability, paresthesia, and mental disturbances. Rheumatologic symptoms include: joint pain, gout, and pseudogout. GI symptoms include: anorexia, nausea, vomiting, constipation, and peptic ulcers. Additional effects include calcification of the cornea and other soft tissues. Unilateral vocal cord palsy in a patient with hypercalcemia should raise suspicion of parathyroid carcinoma.
Laboratory tests
Laboratory testing for parathyroid carcinoma reflects the presentation of hyperparathyroidism and thus focuses on serum calcium and serum PTH measurements (see Chapter 10 : Disorders of Calcium Metabolism). In benign causes of hyperparathyroidism serum calcium concentrations are typically in the range of 10–11 mg/dL, while in malignant cases concentrations are typically higher, in the range of 15–16 mg/dL. Ten percent of patients have hypercalcemic crisis defined as rapid-onset serum calcium >14 mg/dL and signs or symptoms of multiorgan dysfunction. Associated intact PTH concentrations are also markedly above the reference range (3–10 times). Another manifestation of hyperparathyroidism is increased urine calcium excretion (>250 mg/day). The utility of intraoperative PTH during surgical resection for parathyroid carcinoma is unclear due to the small number of reports.
Tumor imaging and surgery
Parathyroid tumors can be imaged with ultrasonography, CT, and MRI and imaging modalities can be useful adjuncts for diagnosis and for detecting metastases. Suspicion for carcinoma is raised with a large parathyroid lesion (>3 cm) or bone and kidney involvement. Technetium-99m sestamibi scintigraphy is useful for localization as opposed to distinguishing benign and malignant lesions. Preoperative suspicion is essential for the best chance at curative surgical treatment and to reduce the risk of recurrence. Open bilateral exploration with complete resection that avoids capsular disruption with en-bloc removal of the tumor and adherent tissues and the ipsilateral thyroid lobe is recommended. Follow-up includes serum calcium and PTH measurements and neck ultrasound.
Thyroid
Epidemiology
A total of 52,890 new cases of thyroid cancer and 2180 deaths from thyroid cancer are projected for 2020. Thyroid cancer is overwhelmingly the most common endocrine malignancy. Cases of thyroid cancer increased rapidly at 7% per year throughout the 2000s likely due to increased detection from imaging and sensitive diagnostic procedures. Rates stabilized in women by 2016 and were down to2% in men perhaps as a result of more conservative diagnostic criteria. The incidence rate is three times higher in women compared to men. It is the leading site of new cancers for ages 15–29 years. In hereditary MTC the peak incidence is between ages 10 and 30, while the peak incidence is between ages 40 and 60 for sporadic MTC. The 10-year survival in patients with localized MTC is >90%, decreasing to 78% and 40% in patients with regional or distant metastases, respectively.
Risk and hereditary factors
Thyroid nodules are common, with an estimated prevalence of 5% although occult nodules discovered incidentally on imaging is estimated at 19%–68% in the general population. Approximately 90% of nodules are benign and 95% of individuals are asymptomatic. The risk of thyroid cancer increases with age and with female sex. A major risk factor is exposure to radiation in childhood primarily from treatment, but also exposure to ionizing radiation from fallout from atomic weapons or nuclear power plant accidents. Five to ten percent of irradiated individuals develop cancer, primarily papillary, and 4%–9% of cancer patients have a history of neck radiation. Risk factors also include a history of thyroid cancer in a first-degree relative or hereditary syndromes such as MEN2, FAP, Carney complex, Werner syndrome, and Cowden syndrome. Twenty-five percent of MTC is familial, resulting from either MEN2A or MEN2B syndromes. The syndromes are inherited in an autosomal dominant fashion and result from mutations in the RET proto-oncogene. The clinical presentation and manifestations of the familial and sporadic disease are similar. Somatic RET mutations are found in 65% of sporadic MTC cases. In at-risk individuals the likelihood of MTC can be mitigated with prophylactic thyroidectomy.
Pathology
Histologic types of thyroid cancer include differentiated: papillary, follicular [conventional and oncocytic (Hürthle cell)], medullary, and undifferentiated (anaplastic). Medullary and anaplastic are more aggressive forms and more likely to metastasize. The most common forms comprising >90% of thyroid cancers are papillary carcinoma (60%–70% of cases) and follicular carcinoma (15%–20% of cases). Papillary tumors are the predominant form resulting from radiation exposure with the associated RET/PTC fusion gene. Mutations in differentiated thyroid cancer are primarily in the mitogen-activated protein kinase (MAP kinase) pathway that regulates cellular proliferation and differentiation with mutations in BRAF V600E in 40% of papillary cancers and 45% of anaplastic cancers. RAS mutations are found in papillary (13%), follicular (40%–50%), benign follicular adenomas (20%–40%), and noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), an early state variant with low malignant potential (30%). TERT (telomerase reverse transcriptase) and TP53 tumor suppressor genes have been found in papillary (10% and <1%) and anaplastic (<1% and >70%) thyroid cancers, respectively.
Medullary carcinoma, arising from the parafollicular calcitonin secreting C-cells, comprises 1%–2% of thyroid malignancies. The sporadic form of the disease is typically unilateral in presentation, while hereditary forms (25%) are bilateral. In familial forms, the transformation to MTC is preceded by C-cell hyperplasia.
Clinical features
The majority of patients with thyroid cancer have no significant symptoms. Occasionally an enlarged neck mass may be present with the sensation of a lump or foreign body in the throat, hoarseness, dysphagia or swallowing concerns, and neck pain. Thyroid masses may be discovered during physical examination or on imaging for a different purpose. Nonpalpable and palpable nodules have the same risk of malignancy. Most cancerous lesions are single nodules with a hard consistency and fixation. Features specific to MTC include: secretory diarrhea in 30% of patients, seen in advanced disease, resulting from intestinal electrolyte disturbances caused by high circulating calcitonin concentrations, flushing, resulting from secretions of prostaglandins and serotonin, and Cushing syndrome as a result of occasional tumor ACTH secretion.
Laboratory tests
Most patients with thyroid cancer are euthyroid and therefore thyroid function tests are not useful for diagnosing thyroid cancer. However, thyroid stimulating hormone (TSH) should be measured in the initial evaluation of a patient with a thyroid nodule, followed by radionuclide scan for focal uptake if TSH is low can aid in the evaluation of thyroid nodules. Hyperfunctioning nodules are rarely malignant. Treatment for thyroid carcinoma depends on factors such as patient age, tumor type and size, and extent of disease, and typically consists of partial or total thyroidectomy and occasionally lymphadenectomy. Radioactive iodine treatment with I 131 may be employed after total thyroidectomy to destroy any remaining thyroid tissue for large tumors or when there is extrathyroidal spread. Targeted therapy with tyrosine kinase inhibitors may also be used in some advanced cancers. Following treatment, TSH concentrations are measured to monitor thyroid hormone therapy. TSH is suppressed by thyroid hormone replacement therapy to suppress regrowth of the tumor and decrease the likelihood of thyroid cancer progression and recurrence. TSH concentrations <0.1 mU/L in patients at high risk and low TSH concentrations in patients at low-risk are general recommendations depending upon treatment and thyroglobulin (Tg) concentrations.
Tg, a 660 kDa dimeric glycoprotein synthesized in the follicular colloid of the thyroid gland, is the precursor protein for thyroid hormone synthesis and is regulated by TSH. Serum Tg concentrations are used as a tumor marker to monitor the clinical course of patients with well-differentiated (papillary or follicular) thyroid cancer following treatment. Most normal euthyroid individuals have detectable serum Tg concentrations. The majority of patients with elevated Tg concentrations have benign thyroid conditions, as Tg is a nonspecific indicator of thyroid dysfunction and concentrations can be elevated in Hashimoto’s thyroiditis, Graves’ disease, thyroid adenoma, and subacute thyroiditis. Therefore Tg is not useful in diagnosing thyroid cancer; however, it is extremely useful for detecting residual thyroid tissue or recurrent disease.
Tg can be measured by radioimmunoassay (RIA), sandwich immunoassay, or liquid chromatography mass spectrometry (LC-MS/MS) with immunoaffinity enrichment. Second-generation sandwich immunoassays on several automated platforms have functional sensitivities of 0.1 ng/mL and allow monitoring for recurrence postthyroidectomy without the need for TSH stimulation from thyroid hormone withdrawal or recombinant human TSH administration. Current RIA and LC-MS/MS assays do not meet the second-generation definition with typical functional sensitivities of 0.5 ng/mL. Tg autoantibodies (TgAb) can interfere in Tg immunoassays, specifically in sandwich assays resulting in an underestimation in Tg concentrations. Approximately 25% of thyroid cancer patients have autoantibodies present at some point during their disease and 10% of normal individuals also have TgAb. TgAb should be analyzed with each Tg measurement to aid in interpretation of results. Similar to other tumor markers, monitoring of individual patients should be performed with the same Tg assay, irrespective of methodology, to facilitate trending and doubling time calculations. TgAb assay results and cutoffs can also vary among manufacturers and cutoff definition (normal population vs assay sensitivity) can make determination of antibody status difficult. Additionally, trends in TgAb concentrations can reflect response to thyroglobulin-secreting thyroid tissue with new or rising TgAb associated with disease recurrence and declining concentrations associated with a favorable prognosis. Use of TgAb as a tumor marker and as an adjunct to Tg measurements further emphasizes the need to follow patients with a specific assay as well as the need for assay harmonization.
Unlike immunoassay, it is thought that LC-MS/MS methods for Tg, which detect Tg-peptides following proteolytic digestion and enrichment with immunoprecipitation, are free from antibody interferences. However, these methods are less well characterized for their ability to accurately assess disease status in thyroid cancer patients. Mass spectrometry methods may be incorporated into reflex strategies for Tg testing, whereby an initial TgAb measurement dictates triage to immunoassay in TgAb-negative specimens and mass spectrometry or RIA in TgAb positive specimens.
Calcitonin is the most important tumor marker for the C-cell derived MTC. In MTC, the production of calcitonin by the follicular C-cells is used to confirm fine needle aspirate findings, to monitor treatment and to detect disease recurrence. Calcitonin concentrations correlate with the extent of disease such as tumor volume and stage. Higher levels are found in palpable disease, while concentrations may be slightly elevated in normal or smaller tumors. Measurement of calcitonin for evaluation of thyroid nodules is controversial; however, basal concentrations >100 pg/mL are suggestive of MTC and concentrations >500 pg/mL suggest local or distant metastatic disease. The sensitivity of calcitonin in detecting small tumors and postoperatively when basal levels are undetectable is increased using stimulation with calcium and/or pentagastrin, although pentagastrin availability is an issue in the United States. In addition to calcitonin, CEA, reflecting tumor burden, is measured pre-operatively as well as serum calcium and fractionated plasma metanephrines to test for hyperparathyroidism and pheochromocytoma. Germline testing for ret proto-oncogene mutations is also performed even without a family history of MTC or MEN2. Postoperative management includes measures of calcitonin and CEA to detect residual or recurrent disease.
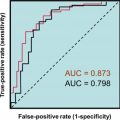
Other tests
Fine-needle aspiration with cytology is a primary diagnostic tool for distinguishing malignant and nonmalignant disease. The 2017 Bethesda classification system stratifies cytological results into six categories with associated risk of malignancy: (1) nondiagnostic (0%–5%); (2) benign (0%–3%); (3) atypia of undetermined significance or follicular lesion of undetermined significance (FUS) (10%–30%); (4) follicular neoplasm or suspicion (25%–40%); (5) suspicious for malignancy (50%–75%), and (6) malignant (97%–99%). Categories 3 and 4 are considered indeterminate, as follicular cancers and adenoma are unable to be distinguished, thus usually resulting in additional evaluation including molecular testing, commonly mutational analysis, and gene expression analysis or diagnostic lobectomy. In differentiated thyroid cancer, fine needle aspiration (FNA)-Tg washout can be useful as an aid to cytology in detecting lymph node metastases in patients with the greatest utility in patients who have undergone total thyroidectomy. Cytomorphologic examination can be nondiagnostic, most often due to insufficient cellularity. Following ultrasound-guided fine-needle aspiration of the suspicious lymph node, the needle is rinsed with saline or buffer and the sample tested for Tg. Detectable Tg is suggestive of the presence of metastatic disease; however, there is no uniform diagnostic cutoff for a negative result, as sample collection methods and assays are not standardized.
Immunohistochemical stains for calcitonin, CEA, and neuroendocrine markers can be used to improve the sensitivity of FNA for the diagnosis of MTC. Similar to FNA-Tg washout, measurement of calcitonin in suspicious lymph nodes following treatment may have utility, although laboratories performing this testing are limited.
Tumor imaging
Ultrasound and radionuclide scans are often part of the diagnostic workup of thyroid nodules. Ultrasound is used for cancer risk stratification and decision to undergo FNA biopsy. Scoring systems have been developed based on sonographic characteristics to assign risk for malignancy. In MTC, postoperative imaging may include neck ultrasound in patients with small elevations in calcitonin (>20 pg/mL) and more extensive imaging in patients with calcitonin concentrations >100–200 pg/mL.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree