CHAPTER 37 Malaria
Introduction
Malaria is a devastating infection that affects over 500 million persons annually, causing more than 2 million deaths.1,2 Severe morbidity and mortality is predominantly inflicted upon children, particularly those less than 5 years of age in sub-Saharan Africa. It has been estimated that a child dies, on average, every 30 seconds due to this protozoal infection. Despite recent attention malaria continues to plague the developing world and is particularly prominent in areas with limited resources. Immigrants and refugees are increasingly migrating from areas of high malaria endemicity such as sub-Saharan Africa. Unfamiliarity of medical practitioners in nonendemic areas with malaria can lead to delays or errors in diagnosis and appropriate treatment.3
Malaria life cycle
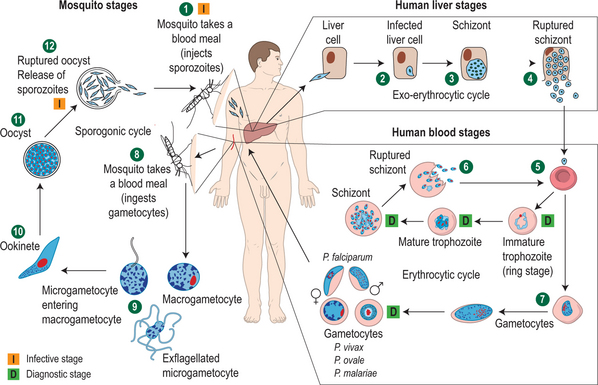
Human malaria is caused by four species of the plasmodia parasite: Plasmodium falciparum, P. vivax, P. ovale, and P. malariae. The parasites, in the form of sporozoites, are introduced into the blood through the bite of a female Anopheles spp. mosquito. The sporozoites remain in the circulation for approximately 30 minutes before they infect a hepatocyte. For the next 7–10 days the parasites develop in the hepatocytes and are then released into the circulation as merozoites which subsequently invade red blood cells (RBCs). The host does not become clinically ill until the posthepatic phase. Both P. vivax and P. ovale can become dormant in the hepatocyte, in a form referred to as a hypnozoite. The hypnozoite may emerge months to years after initial infection to cause disease. Signs and symptoms of malaria caused by P. falciparum, which does not possess the hypnozoite form, will generally occur within a month of infection (Fig. 37.1).
Epidemiology
Global distribution of malaria
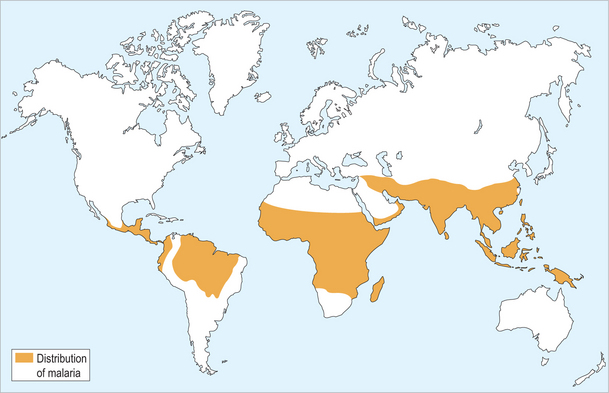
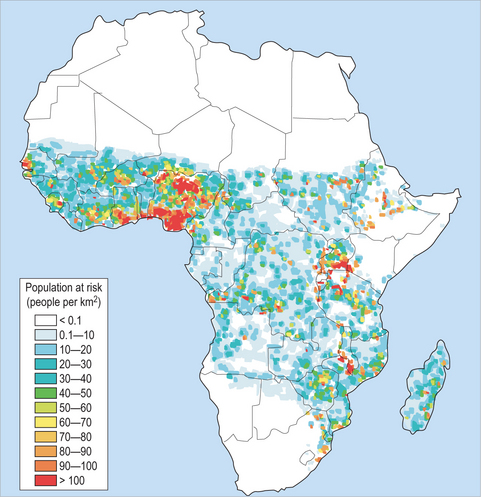
Worldwide distribution of malaria is shown in figure 37.2. The four species of malaria vary widely in their intensity and distribution. Plasmodium falciparum is prevalent in sub-Saharan Africa (Fig. 37.3) but is also found in South Asia, Southeast Asia and Pacific rim islands, South America and small portions of Central America, as well as in Haiti and the Dominican Republic. P. vivax is found predominantly in South Asia, the Middle East, Southeast Asia and Pacific rim islands, and South America, and accounts for a majority of the malaria reported in Central America. P. vivax can also be found in Africa, especially in the Horn of Africa and south along the eastern coast. It is interesting to note that it is an unusual infection in a majority of sub-Saharan Africa, in many areas accounting for less than 10% of cases. This scarcity of P. vivax is thought to reflect evolutionary genetic loss of an antigen on the red blood cell surface termed the Duffy group antigen. The parasite must attach to this antigen to infect a red blood cell and continue its life cycle and, due to its absence in many sub-Saharan African populations, the parasite is unable to cause disease. P. ovale is a less common malaria parasite which is restricted to Africa, predominantly West Africa, while P. malariae is also found mostly in Africa.
The clinical manifestations of P. falciparum malaria depend on many factors including epidemiologic patterns and immune status of the exposed individual. Nonimmune individuals including infants, young children, and persons residing outside of holo- or hyperendemic malarious areas (i.e. travelers) are at the greatest risk of severe P. falciparum malaria. Although several species of malaria infect humans, the burden and consequences of P. falciparum predominate. P. falciparum may lead to death in a nonimmune host less than 12 hours from onset of symptoms.4 However, in highly endemic (holoendemic) areas more than 75% of the population may be affected at any given time, with a majority of individuals being asymptomatic due to acquired partial immunity. These variations of clinical disease and asymptomatic infection may, in part, be due to gene expression variation between serotypes of P. falciparum.5 Areas with high malaria endemicity include a majority of West, Central and portions of East Africa. While the less deadly, relapsing forms of malaria (P. vivax, P. ovale) occur in sub-Saharan Africa, these infections account for a minority of infections.
Other areas such as Central Asia, South Asia, Southeast Asia, and areas of Latin America and the Caribbean have varying degrees of malaria, although in these areas malaria rarely reaches hyper- or holoendemic rates. These areas also have varying ratios of deadly P. falciparum and nonfalciparum, although most areas outside sub-Saharan Africa have higher percentages of nonfalciparum malaria, particularly P. vivax.
Malaria and the immigrant to the United States
Malaria was endemic in most of the continental United States and Europe into the nineteenth century with sporadic outbreaks as far north as Minnesota. There are several species of Anopheles mosquitoes (especially Anopheles quadramaculus) endemic to the US capable of transmitting malaria under favorable conditions. There have been a number of recent autochthonous cases and small outbreaks of malaria transmitted in the US including both P. falciparum and P. vivax.6,7
There is concern that resettling immigrants and refugees, especially those from areas of high endemicity, may act as potential reservoirs for malaria. Although sustained malaria transmission would be unlikely, single autochthonous cases or small outbreaks would be possible. This concern is further exacerbated by data recently collected which show that refugees may become clinically ill from P. falciparum over 6 months after the last exposure-indicating active infection, and parasitemia for prolonged periods of time after arrival (unpublished data, William Stauffer) suggesting subclinical circulating parasites. As stated, P. falciparum may be extremely virulent in nonimmune hosts and autochthonous transmission could lead to severe consequences. Partially due to this concern, in the late 1990s, the Centers for Disease Control and Prevention (CDC) recommended that all refugees departing for the US from sub-Saharan Africa receive presumptive therapy for malaria. These recommendations were implemented in May of 1999 when the International Organization of Migration (IOM) began presumptive treatment of refugees with sulfadoxine-pyrimethamine (SP, Fansidar®). Data collected prior to 1999 (1997–1999) found that >60% of Liberian refugees, arriving from four primary countries of asylum in West Africa, had active infection (parasitemia) 4 weeks after arrival in Minnesota.8 In another study of refugees arriving in Canada from the less endemic area of Tanzania, it was found that 18% of refugees three months after arrival had evidence of active infection.9 Since the implementation of the pre-departure presumptive antimalarial treatment few data are available on malaria imported to the US by newly arriving immigrants or refugees. One study, performed in 2004, compared a rapid antigen test to a single thick and thin blood smear using polymerase chain reaction (PCR) as the gold standard. When 103 newly arrived Liberian refugees were tested 4 weeks after arrival, 8.7% had active P. falciparum infection.10 Therefore, although the implementation of the empiric treatment program seems to be associated with a substantial decrease in the prevalence of active malaria in West African refugees, it does not eradicate the disease from the population. The authors postulate this is due to one, or more, of the following factors: drug-resistant P. falciparum with recrudescence of disease (rates of >50% SP resistance have been documented in Freetown, Liberia), failure to receive the medication (although IOM reports 96% treatment rates), and/or reinfection after treatment prior to departure. In 2004, the CDC modified the recommendation for presumptive pre-departure treatment from SP to artemisinin combination therapy due to concern regarding SP resistance. Due to cost concerns and a shortage of artemisinin coagulation therapy, this strategy has not been implemented.
A recent study, at a single county hospital and clinic system in Minnesota, further demonstrates the success of the presumptive therapy program.11 Prior to 1999, the African refugees arriving in the county experienced rates of 6–9% per year of presentation of clinical malaria, costing this single county hospital ≈$75 000–100 000 per year. Since 1999, the percentage of African refugees who subsequently develop malaria has dropped to 0–2% per year, despite a dramatic increase in the number of African refugees arriving in the county since 1999–2000. In this study it was estimated that for every 15 African refugees treated with presumptive antimalarial therapy prior to departure, one case of clinical malaria is prevented in the United States (number needed to treat = 15).
Clinical Manifestations
Common malaria presentations in immigrants
Plasmodium falciparum generally presents days to weeks after initial exposure; however, in the partially immune population it may present several months or more after arrival. The classic clinical presentation of P. falciparum malaria is high fevers accompanied by chills, rigors, sweats, and headache. Malaria is also commonly associated with generalized weakness, backaches, myalgias, vomiting, pallor, jaundice, and hepatosplenomegaly. However, it is important to note that malaria in partially immune individuals may produce no or minimal clinical symptoms. Children frequently present with a syndrome that includes vomiting and/or diarrhea that may be confused with gastroenteritis. Other common presenting signs and physical symptoms in new immigrants include anorexia and decreased activity in children, isolated splenomegaly with or without a protuberant abdomen, or fever alone. A high clinical suspicion of malaria must be maintained when working with a population from a country where malaria occurs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree