Introduction
Cancers are largely diseases of ageing. The most recent SEER (Surveillance, Epidemiology and End Results) summaries through 2006 show that 61% of all cancers occur in people over age 65 years, an important fact for Medicare in the USA and similar health insurance programmes elsewhere. Age-specific rates continue to rise well into the ninth decade of life. Even though cancer mortality in the elderly is also higher and 71% of all cancer deaths occur in people of Medicare age, there are nonetheless 6.5 million cancer survivors over age 65 years, of whom 4.4 million are long-term, that is, >5 year, survivors. Therefore population studies of cancer and cancer survivorship are studies of ageing.1, 2
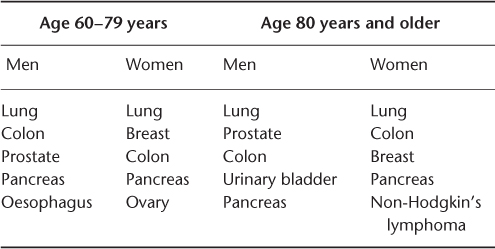
Cancer is the leading cause of death for men and women aged 60–79 years, followed by heart disease. This is reversed among those aged 80 years and over, with heart disease causing more deaths than cancer.3 Table 111.1 shows that cancer as a cause of death declines in importance relative to cardiovascular diseases in advanced old age.3 As shown in Table 111.2, among the 10 most common cancers affecting adults, by a wide margin breast, prostate, lung and colon cancer occur mostly among the elderly.
Table 111.1 Five leading causes of death: US men and women aged 60 years and older, 2009.
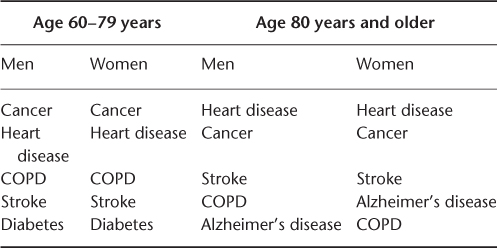
Table 111.2 Five most common sites of cancer mortality: US adults, 2009.
In Table 111.3, it can be seen that the common cancers have good prospects for prolonged survival. All except lung cancer, if detected at an early stage, have better than a 50% 5 year survival.3 Therefore, the goal of optimizing physical and mental function for elderly cancer patients and survivors affects hundreds of thousands of people. Treatment protocols tested in clinical trials have included few if any elderly participants over age 70 years, so individualizing cancer treatment for elderly patients requires individualizing functional assessments also.4
Table 111.3 Percentage 5+ year survival by cancer site, 1996–2004.
| Cancer site | Survival rate (%) |
| All cancers | 66 |
| Prostate | 99 |
| Breast | 89 |
| Urinary bladder | 81 |
| Rectal | 67 |
| Colon | 65 |
| Non-Hodgkin’s lymphoma | 65 |
| Ovarian | 46 |
| Oesophagus | 17 |
| Lung and bronchus | 16 |
| Multiple myeloma | 16 |
| Pancreas | 5 |
A Role for Geriatricians in Cancer Care
There is a strong referral bias for more fit elderly people in cancer clinical samples.5 Clinical decision-making in cancer is further complicated by marked under-representation of elderly persons in cancer clinical trials.6 Analyses of population treatment data through the linked SEER–Medicare database suggest that frail elderly people are unlikely to be referred to cancer specialists in community practice. Hurria et al.7 performed standard geriatric functional screening on elderly CALGB trial enrollees and found that functional impairment defined as any ADL (activity of daily living), IADL (instrumental activity of daily living) or cognitive deficit was rare. For example, Gupta and Lamont8 showed that the proportion of elderly with a diagnosis of dementia was lower among Medicare beneficiaries treated for colon cancer than in the general population. More recently, Lamont et al. used the SEER–Medicare database to compare treatment outcomes in community practice with those of trial participants and found that poorer results in the community treatment cohorts were not entirely explained by comorbidities.9 Either trial participants were fitter regardless of comorbidity or receiving treatment through a trials centre conferred better clinical management.
Balducci adapted the consensus frailty phenotype as defined by Fried and co-workers’10, 11 to making decisions about cancer therapy.12 He included ADL and IADL disability, non-cancer severe comorbidity and presence of ‘geriatric syndromes’ including cognitive impairment, falls and delirium as probably excluding an older cancer patient from receiving full dose or any chemotherapy.12 Further, he effectively explained in terms of cancer-related life expectancy why frail elderly patients may be harmed with highly toxic therapy. There appears to be a tacit agreement in community practice not to subject obviously frail and otherwise incapacitated elderly people to toxic therapy.
The role of geriatrics in these decisions is to identify vulnerabilities that are not evident in apparently well elderly subjects, to prescribe and provide supportive interventions for patients with good prognosis cancers, to participate in the transition from disease control to symptom palliation and to communicate clearly with patients and families when the trajectory of disease is approaching end-of-life.
Additionally, geriatricians inevitably care for long-term cancer survivors. Survivorship care entails awareness of cancer-specific sequelae. Survivors experience continuing adverse health for years after the event compared with those who never had a malignancy.13, 14 Ganz and Hahn15 and others16, 17 therefore proposed specific survivorship care plans that go beyond mere surveillance for recurrences.
Functional status as used by geriatricians refers to activities of daily living (ADL), the ability to care for oneself at home, and instrumental ADL (IADL), the ability to live alone and manage one’s own household affairs. This is different from oncologists’ construct of performance status, which has more to do with exercise tolerance, grading activity levels from fully physically active outside the home to bedbound. Using a summary Karnofsky Performance Score (KPS)17 or Eastern Cooperative Oncology Group Performance Score (ECOG-PS), oncologists make very accurate predictions about survival and ability to tolerate toxic therapies. Summary KPS or ECOG scores, however, are relatively insensitive to risk for functional decline and fail to identify the so-called vulnerable elderly. The summary scores do not identify specific functional disabilities that might be reversible, nor do they suggest how that might be done.18 A short functionally based screening such as the ACOVE VES-13 has been proposed as a quick way to select apparently fit elderly cancer patients for further evaluation.19, 20 A more extensive battery of screening tools has been shown to be feasible to perform in the outpatient oncology setting.7 Several studies have suggested that abbreviated geriatric measures of function provide actionable data.21 For example, Extermann et al. addressed fall risk reduction for breast cancer patients.22 Bylow et al. established a high prevalence of previously under-reported falls among prostate cancer patients on hormonal deprivation therapy.23
There have yet to be any randomized trials to test whether routine geriatric assessment could improve patient tolerance of cancer therapy. Cohen et al. reported a randomized clinical trial of continuity of care for geriatric veterans who received inpatient geriatric assessment and intervention with follow-up in outpatient GEM (geriatric evaluation and management) and home-based care.24 Post hoc subgroup analysis revealed that older veterans with a cancer diagnosis benefited the most from geriatric continuum of care. Although they did not live longer, quality of life measures were statistically significantly improved.25
Staging the Ageing of the Elderly Cancer Patient
Cancer treatment often involves sequencing multimodal interventions including surgery, chemotherapy and radiation. There are several key aspects of geriatric assessment that are particularly salient for patients and physicians planning surgical cancer treatment. In addition to standard preoperative risk stratification, preoperative assessments should be able to anticipate whether subacute care26, 27 at home or at a long-term care facility (LTCF) will be needed. The goal is to prevent SNF placement but if it becomes necessary, selecting a LTCF or SNF (skilled nursing facility) on the day of discharge is disconcerting for families and patients. Since it is usually predictable, shopping for acceptable facilities should begin early. If surgery will involve the head and neck or a long intubation, facilities’ capacity to provide specialized oral care and speech and swallow therapies should be considered carefully, not just their ability to provide tracheostomy care. Nutritional support and evaluation are required to re-establish oral feeding.28 Furthermore, early feeding when possible with use of protein–calorie supplements have been shown to improve surgical outcomes in elderly surgical patients.29 Cancer surgery outcomes for the elderly are improved by early mobilization and early nutritional support.26, 27
Use Structured Methods to Establish Decisional Capacity Prior to Treatment
If chemotherapy is contemplated, geriatric assessments should be performed proactively to determine the patient’s decisional capacity, that is, whether the patient is cognizant of the risks and benefits of the alternative courses. If there is any doubt, formal evaluation of decisional capacity is mandatory.30 If decisional capacity is impaired, this should be factored into treatment decisions since at all stages of disease, cognitively impaired patients fare substantially worse.8, 31 Unrecognized cognitive impairment32 and delirium33 are common in elderly cancer patients. The complexity of the decision to be made needs to be titrated to the patient’s cognitive capacity. There are four established legal standards for determining capacity: the patient is aware of the treatment options and expresses a treatment preference clearly and consistently; the patient can give a conventionally understandable reason that is consistent with their past behaviour; the patient retains and reproduces the information given as the context for the decision; the patient understands what their decision means for their own state of health.30
Figure 111.1 shows a schematic diagram of the decisional standard required to consent to each level of therapy offered. Physically robust, cognitively intact older patients have been shown to derive equal benefit from equal treatment in clinical trials and should not therefore be excluded from standard therapy and clinical trials on age alone.6, 34–36 Therefore, staging the ageing is as important as staging the cancer.
Figure 111.1 Matching the complexity of clinical decision-making to the decisional capacity of the patient.
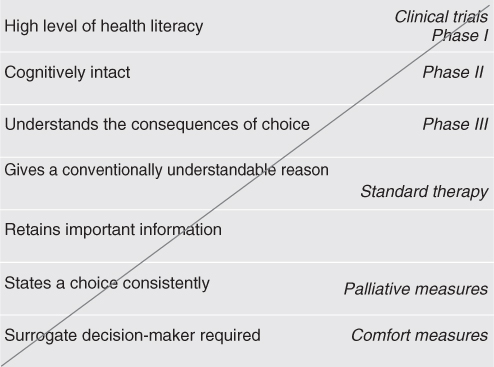
The diagonal axis in Figure 111.1 describes the ratio of risk to benefit for the course of treatment. The hierarchy of legal standards used to determine decisional capacity is arrayed on the y-axis. Usual care and clinical trials are marked on the diagonal suggesting the standard required for consent. Cancer care options are inclusive from least to most risky.
The short term impact of chemotherapy on functional capacity should be assessed proactively. Is the patient at risk for delirium? This bears directly on the patient’s ability to self-manage chemotherapy-associated toxicities, medications and nutrition. What is the patient’s home support system? An elderly person living alone who manages fairly well in their usual state of health is judged fit for chemotherapy by having an ECOG-PS of 2 or less. They are likely to do well in the infusion suite but may develop problems with delayed toxicities. It is critical that spousal, family or pre-emptive home care supports be put in place to prevent unplanned hospitalizations due to falls, delirium, malnutrition and volume depletion. The National Cancer Care Network (NCCN), which represents the largest number of community-based cancer treatment centres, has adopted these recommendations as part of its body of treatment guidelines for specific cancer sites in the elderly. An extensive summary statement of the evidence for these guidelines has been prepared by the International Society for Geriatric Oncology (SIOG) based in Geneva.18
Although numerous studies have reported that fit elderly patients tolerate cancer therapy without undue acute toxicities, elderly cancer patients have several specific chemotherapeutic risk factors. Dosing must be adjusted for reduced renal and hepatic function. Still, it has been well described that elderly patients experience more severe and more prolonged neutropenia from cytotoxic agents. Although studies are not entirely consistent, the NCCN has also recommends prophylactic use of granulocyte colony-stimulating factors to prevent neutropenia in elderly patients based on a high incidence of severe bone marrow toxicity observed in the elderly in clinical trials and increased risk for sepsis.37, 38 With the high risk for delirium, the use of ‘prn’ (‘when necessary’) advice to an elderly patient living alone, even with ‘drop-ins’, is simply not adequate. Outpatient management must prevent hospitalizations for toxicities which can spiral into loss of functional capacity and nursing home placement. Elderly cancer patients should be vigilantly screened for risk of delirium and clinical suspicion should be high.32
Giving chemotherapy to a long-stay resident in an LTCF presents an array of complex medical decisions and ethical concerns. However, short-stay SNF residents may be receiving outpatient chemotherapy and radiation and the responsible nurses and physicians need to know what to expect. Hence transitions of care for elderly cancer patients are fraught with danger of communication lapses.39 This is especially true since SNF care assumes, indeed based on the reimbursement codes, requires, functional improvement. Without a correct and clearly communicated cancer prognosis at the time of transfer, appropriate care cannot be given. Physician-to-physician communication by telephone, electronic record-sharing or writing is imperative. Prognostic errors go both ways, unrealistically optimistic and pessimistic, on both sides. The idea that an elderly patient with any malignancy is hospice appropriate can be as mistaken as the idea that a patient with an untreatable advanced malignancy can be functionally upgraded.
It often falls to the long-term care physician to state what was not clearly understood at hospital discharge. Over 65% of oncologists report that they do not routinely discuss prognosis, advance directives or end-of-life until the patient is within days to weeks of death. This contrasts with younger oncology physicians who report having these discussions before the need.40 There is an interesting correspondence with patient preference in this study. A similar >60% of cancer patients preferred not to have these discussions with their oncologists; rather, they expressed no unwillingness to discuss advance directives and end-of-life with hospital doctors, which means typically hospitalists and house staff.40
Supportive Management During Cancer Treatment is Just Good Geriatric Care
Supportive oncology is the branch of palliative medicine that addresses the management of symptoms due to cancer and to the debilitating effects of cancer treatment with the goal of maintaining patients’ quality of life. All major cancer centres have invested in supportive care because it offers the best chance for patients to be able to complete treatment. When treatments fail and cancer progresses, supportive oncology manages the transition to hospice.
Four randomized clinical trials have compared palliative care delivered with cancer treatment to usual care with optional palliative referral as determined by the treating physician. Patients with advanced cancer in rural Vermont, N = 322, mean age about 65 years, were randomized to psychoeducational intervention with monthly telephone follow-up by advanced practice nurses. At the end of the study, quality of life and mood scores were higher in the intervention group, but there was no difference in symptom intensity or hospital days.41 Two additional trials also showed improvements in self-reported quality of life among patients randomized to palliative care along with usual cancer care, but the differences were not statistically significant.42, 43 Similarly, a Norwegian trial was suggestive but inconclusive.44 A more recent study reported that 151 advanced-stage lung cancer outpatients were randomized to concurrent palliative care or usual care. The mean age was again about 65 years. Mean change scores on symptom scales and quality of life scales favoured the experimental group, but the differences were not statistically significant. The experimental group survived on average 2.7 months (30%) longer and used fewer hospital days at the end-of-life.45 One reason for the more definitive findings of this study is that participants were more homogeneous in terms of type and stage of disease and therefore had less variance in clinical course. Previous studies mixed different cancers and stages and lacked statistical power due to small numbers.
As with randomized controlled trials (RCTs) of geriatric interventions, it may not be possible to identify which interventions explain the results. That is, both GEM and palliative care involve multiple disciplines individualizing care, doing different things for each patient. This is a profoundly unsettling model of care for physicians trained to use drug trials of single agents, multidrug protocols in the case of oncology, to decide what works. It is unsettling to rely on questionnaire responses as endpoints over so-called ‘hard’ end-points such as disease progression, hospitalization and death. If similarly designed studies reproduce the finding of less hospitalization and longer survival, it shows that it is feasible to develop ‘harder’ evidence.
In the supporting oncology literature, it is clear that the burden of symptoms and also the stage of disease drive functional status. Targeting the most troublesome symptoms should improve functional status. The caveat in this literature is that the outcome measures, the portfolio of standardized symptom scales, are different from the functional geriatric measures, they are subjective, the numbers are small, patients are not particularly old and a variety of tumour types and stages are reported. The construct ‘quality of life’ includes functional status and a number of other things such as satisfaction, mood and energy. One review enumerated over 100 different definitions of quality of life.46 It is easier to focus on studies of specific symptoms to sort out how treating that symptom affects quality of life, however it is defined. For example, a recently reported RCT of preventive treatment for chemotherapy-associated mucositis showed a significant drop in unplanned hospitalizations in the experimental group who reported less pain and better nutrition.47
In keeping with the purpose of this chapter, to focus on maintaining function and not on end-of-life, the next section addresses specific symptoms that occur during cancer treatment, including pain, fatigue, nausea and vomiting and anorexia. Successful management of these symptoms can make the difference between loss of functional independence due to treatment and obtaining the benefit of treatment. The reader is referred to an excellent summary by Rao and Cohen.48 Geriatricians immediately recognize the fatigue–anorexia symptom complex as age-related frailty. Supportive modalities include pharmacological agents, mind–body alternatives, rehabilitation and cognitive behavioural strategies. The following section looks at randomized trials of pharmacological agents. This is not intended to dismiss non-pharmacological, alternative and mind–body interventions. The level of evidence for these is weak but improving, reflecting the difficulty in designing and conducting trials of complex interventions. Readers are by no means dissuaded from trying them with willing patients.
Follow AGS Guidelines to Treat Pain in the Elderly Cancer Patient
There is not much more to be said about treating pain. People, patients, everyone functions better when the pain is treated. There is no plausible rationale for not treating pain, only that there may be different ways to treat pain and patients may differ in how aggressively they wish to be treated for pain. Cancer pain is complex. It is both nociceptive and neuropathic. There may be components of anxiety and depression. There are specific pain syndromes associated with surgeries, with radiation and tumour invasion plexopathies, bone pain and visceral pain, post-chemotherapeutic neuropathies and oral pain from mucositis. To the specialist, each has an appropriate treatment. Patients with specific surgical pain syndromes should be referred to specialist care, including head-and-neck centres, interventional pain clinics and postoperative rehabilitation centres.
The mainstay of cancer pain management has been the WHO approach to titrating non-opiate and opiate therapy.49 The AGS pain management guidelines focus on how to assess pain in frail or cognitively impaired elderly and on caregiver education to recognize and treat pain.50 Most moderate to severe cancer pain will respond to opiates. In the elderly, the conventional wisdom is to expect a higher peak effect due to a lower number of neural receptor sites (saturation) and a longer duration of action due to slower elimination. An advantage of opiates is the lower likelihood of gastrointestinal (GI) bleeding or renal failure compared with non-steroidal anti-inflammatory drugs (NSAIDS), but NSAIDS are particularly useful for bone pain. There is a higher risk for delirium, falls, nausea and constipation with opiates. In general, opiates with complex metabolism and active metabolites should be avoided, including meperidine, propoxyphene, fentanyl patches and methadone.50 The geriatric practice of choosing the least number of agents by the least invasive route at the lowest dose is good advice with opiates. In general, short-acting agents are to be preferred, such as immediate-release morphine, oxycodone and hydromorphone, titrated under close scrutiny, especially for patients with impaired executive and short-term memory function. Opiates should always be accompanied by a bowel regimen to prevent constipation. Sennosides with stool softeners and osmotic agents together are preferred. Severe opiate bowel may precipitate hospitalizations. Methylnaltrexone is an injectable which blocks mu-opiate receptors in the bowel that does not cross the blood–brain barrier. It can be used for bowel rescue when oral agents fail.
Once the best level of pain relief versus sedation has been achieved, short-acting agents can be converted to a long-acting agent with breakthrough coverage using one of many available conversion tables or calculators. Adjuvants for neuropathic pain should be selected for lowest anticholinergic burden and evidence-based supporting literature. Consideration should be given to topicals, such as lidocaine gels and sports creams. Therapeutic massages, heat, cold, oral rinses and focused physical therapy all have a role. For example, moderate weight lifting proved superior to usual protective advice in reducing post-mastectomy lymphoedema pain in middle-aged women without affecting the actual volume of fluid retained.51
Treat Fatigue in Elderly Cancer Patients to Limit Functional Decline
Considerable research has gone into understanding the non-pain symptoms of cancer and cancer therapy. Whether fatigue is the cause or the effect, elderly cancer patients who report extreme fatigue also report poor nutritional intake, poor sleep, immobility and loss of functional capacity.52, 53 Extreme fatigue is often a reason given for terminating cancer treatment. There are no specific diagnostic features to distinguish the fatigue of cancer from the fatigue of primary frailty. Both are characterized by unregulated cytokine production.54 However, cancer fatigue generally dates to the clinical onset of cancer or to cancer treatment. Students of fatigue describe a multicomponent disturbance of neuroendocrine regulation, cytokine peripheral and central effects including depression and diurnal cycle dysregulation.53 The association between cancer fatigue and anaemia is not as strong as initially thought, although severe anaemia certainly exacerbates fatigue. It has also been difficult to show a simple association between stage of disease and severity of fatigue or between specific treatment modalities and fatigue.53 A follow-up study of clinical trial participants found that fatigue persisted years after the completion of presumably successful cancer treatment.55 There is no consistent association between cancer fatigue and age.52, 53
Fatigue outranks pain as a symptom for cancer patients.52, 55, 56 It is a common complaint among frail elderly people who do not have cancer. Fatigue has been hard to define and hard to treat as it is embedded in a complex cluster of symptoms and disease-related perturbations. It has to be evaluated in the context of the disease trajectory and untangled from cancer-related anaemia, cancer treatment-related anaemia, radiation sickness and radiation-induced hypothyroidism, depression, disturbed sleep and undernutrition. Nonetheless, clinicians believe that it is a distinct cytokine-related syndrome. Studies have tried to define it and measure it so that it may be quantified. NCCN adopted a narrative definition: ‘a persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion (related to cancer or cancer treatment) that is not proportional to recent activity and that significantly interferes with usual functioning’.57, 58 Provisional ICD-10 criteria for the E&M code include six of the following complaints: weakness, heaviness in the limbs, subjective problems with concentration and short-term memory, no motivation to participate in activities, a subjective sense of having to struggle to get moving, not getting the usual daily tasks done, feeling bad rather than pleasantly tired for several hours after exercise, sleeping too much or not enough and never feeling refreshed after sleep.59 Minton et al. reviewed available standardized, validated fatigue screening and severity scales.60, 62
Fatigue is worsened by depression. Depression is underdiagnosed among cancer patients.61 Mor et al. have shown that elderly breast cancer patients are less likely to suffer from new onset depression than younger breast cancer patients.62 However, in evaluating an elderly patient with fatigue, depression should be addressed routinely and explicitly.18
Symptomatic anaemia is treatable and should be treated in elderly patients, particularly those with underlying coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD) or increasing exertional dyspnoea. In one study, correction of anaemia with erythropoietin improved FACT-Fatigue scores but only if FACT-Anemia was also positive.63 Recent clinical studies have dimmed hopes that erythropoietin supplementation for cancer-associated anaemias would solve the fatigue problem and have additional functional and survival benefits.64 Transfusion or correction above 9–10 g dl−1 offered no further symptomatic improvement and increased the risk for thromboembolic and cardiac events.65
Research on the treatment of fatigue has focused on psychostimulants. The best studied is methylphenidate. A double-blinded placebo-controlled randomized trial of short-acting methylphenidate in cancer patients demonstrated improvement and maximum effect within 8 days and a sustained effect over the 4 weeks of the trial that was durable for up to 6 months on drug as measured by the Brief Fatigue Inventory (BFI). However, there was no statistically significant difference between the treated arm and the placebo arm except among patients with BFI-rated >6, severe fatigue.66 Sustained-release methylphenidate showed benefit equivalent to short-acting methylphenidate in a trial enrolling patients undergoing cancer treatment and was also not different from placebo except among the subgroup of patients with BFI >6, severe fatigue.67 A head-to-head comparison of methylphenidate and modafinil favoured methylphenidate.68 Two trials have been reported that tested whether donepezil effectively addresses the cognitive component of cancer-related fatigue. Patients treated with donepezil reported 56% improvement of fatigue symptoms, not different from placebo.69 A randomized trial of placebo versus ‘no-cebo’ reported 56% improvement of fatigue symptoms on placebo versus 36% on ‘no-cebo’.70 In summary, if there are no contraindications, methylphenidate appears to be safe for a trial of treatment for severe cancer-related or cancer treatment-related fatigue in elderly patients.
Anticipate Anorexia, Recognize Cachexia and Support Nutrition
Anorexia means loss of appetite. Loss of appetite and distortions of taste commonly occur in cancer patients and it is critical to determine whether the symptoms are due to the cancer or to cancer treatment. If anorexia causes failure to eat and weight loss, and the patient is not at end-of-life due to the cancer, it is reasonable to try to stimulate appetite and nutritional intake.29, 71 The key differential diagnosis is to recognize cancer cachexia, a cytokine-mediated hypercatabolic state in which both fat and muscle are degraded. Cytokines have central effects on appetite and peripheral effects on metabolism.72–74 Cancer cachexia, unlike protein–calorie starvation, is not reversible solely with nutritional interventions.75 Some cancers induce cachexia early in their course and treating the cancer may induce a brief period of remission with improved appetite and possible modest weight gain. Pancreatic cancer is highly inflammatory and is one such example. Among the common solid tumours including non-small-cell lung, prostate, breast and colon, rapid weight loss occurs late in advanced disease and signals impending death. Haematological malignancies in general do not precipitate cachexia, although immunotherapies may.
In starvation, resting energy expenditure declines and metabolism slows to divert available calories to high-priority end-users such as the brain and heart. Patients with very poor intake can sometimes maintain a remarkable level of physical activity. Hence it is crucial to differentiate weight loss due to cachexia from just poor nutritional intake with or without anorexia. In cancer patients, there may be physical causes for not eating even if hungry. Mucositis, radiation oesophagitis, diarrhoea and nausea, surgical interruption of the GI tract, pain, sedation and drug effects affect eating. Families are often more anxious about disturbances of appetite than the patient in the author’s experience. Treating proximal symptoms can improve intake. High caloric density snacks and liquid protein–calorie supplements are easy to consume and not over-filling.
Anorexia has been the subject of a number of randomized clinical trials. There are four basic classes of orexigenics. Corticosteroids, dexamethasone 4 mg 1–4 times per day or prednisone 5–10 mg per day initially induces a mild euphoria, suppresses nausea and may increase appetite. The risks include further immune suppression, Cushingoid changes with long use, myopathies, HPA (hypothalamic–pituitary–adrenal) axis suppression and hyperglycaemia.76 There is no convincing evidence that they cause weight gain with short-term use and the weight gained with prolonged use is largely fat and water. The progestationals are the best studied orexigenics. These include megestrol acetate 400–800 mg per day or medroxyprogesterone 500 mg twice daily.76 As shown by Yeh et al. in an RCT that did not include cancer patients, frail veterans did gain weight and the maximum effect was seen by 12 weeks of treatment. After 1 year there was no difference in the weight of the experimental and control groups.77 On treatment, participants had lowered cytokine levels.78 There is an increased risk of venous thromboembolism with progestationals and potential for HPA suppression, so they should be tapered once the treatment goal has been reached or stopped if no response is obtained after 12 weeks.
Cannabinoids include medical marijuana, which is not usually considered for elderly patients regardless of diagnosis, and dronabinol 2.5–20 mg twice daily. One widely cited study of advanced HIV patients showed improved appetite but no weight gain after 1 year.79 A head-to-head comparison of megestrol acetate (MA) and dronabinol in 469 cancer patients was reported. The 73% of patients randomized to MA reported better appetite and 13% gained >10% of their starting weight compared with 47% and 3%, respectively, in the dronabinol group. There was no added benefit to combining the drugs and the older patients did not tolerate the dronabinol.80 Recent reports suggest a role for the novel GI peptide ghrelin in managing cancer-related anorexia.81, 82 A number of novel agents, including SARMs (selective androgen receptor modulators) and anti-TNF (tumour necrosis factor) antibodies, have been studied in small numbers of subjects and second-look studies of atypical antipsychotics, thalidomide and anabolic steroids including oxandrolone are under way, but so far definitive evidence is lacking.83
Anticipate and Prevent Nausea and Vomiting
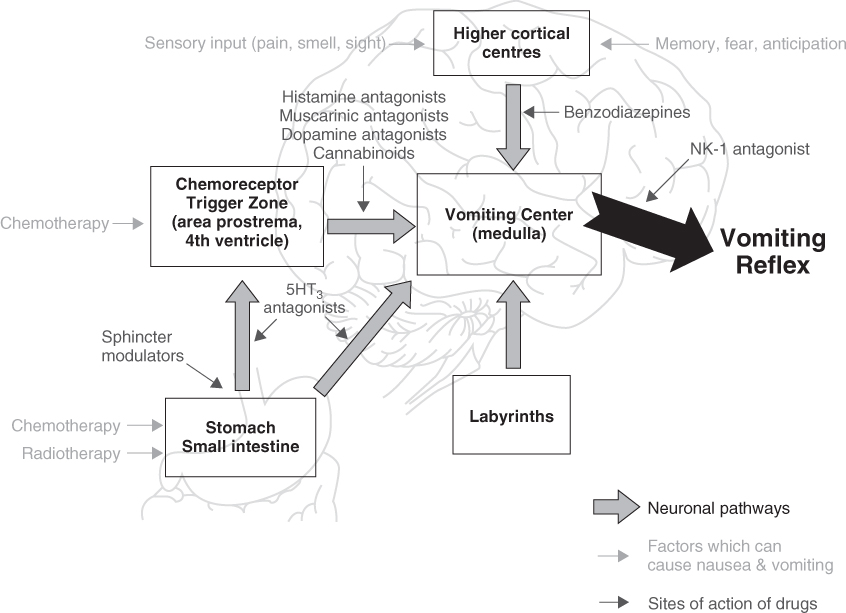
Nausea and vomiting are a normal part of the host defence system among omnivores and carnivores that is not shared by ungulates. It is designed to rid the body of toxic ingested substances. The physiology of nausea is shown in Figure 111.2. The area postrema at the base of the fourth ventricle where the blood–brain barrier is permeable provides early central sensing of toxins in the bloodstream. Stimuli are forwarded to the vomiting centre in the medulla to coordinate the vomiting reflex with feedback to the upper GI tract. Central causes of nausea and vomiting include increased intracranial pressure, vestibular toxicity, motion sickness, acid–base disorders, electrolyte disturbances, chemotherapy and anxiety, so-called anticipatory nausea and vomiting. Elderly patients have been described as less likely to suffer from anticipatory nausea and vomiting than younger patients.84 Many anticancer drugs cause nausea and vomiting.85 Opiates are also highly likely to cause nausea and vomiting, particularly in the opiate-naïve patient and when doses are rapidly escalated.
Chemotherapeutic agents are ranked as minimally to highly emetogenic and infusion therapists generally incorporate premedication into the protocol to prevent acute chemotherapy-induced nausea and vomiting (CINV).85 Perhaps because of less anxiety or because of the selection of less toxic chemotherapeutic agents, elderly cancer patients appear to suffer less with acute CINV. Delayed CINV is also well described and it is a more serious problem for elderly cancer patients. Around 4–10 days after chemotherapy, it is caused by a combination of central and peripheral disturbances and by direct GI tract toxicity. Specific toxicities include oral and pharyngeal pain, digestive endothelial mucosal injury due to cytotoxins and antimetabolites, radiation, increased vagal tone, autonomic failure, hiccups associated with phrenic nerve and diaphragmatic irritation. Gastritis, gastric reflux and gastroparesis cause nausea. These problems are often accompanied by severe diarrhoea and a high risk for volume depletion, falls, delirium and injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree